Abstract
Learning anatomy in medical school is still closely based on the use of cadavers. The burial of preserved cadaver poses a problem, specifically, it contaminates the soil with formalin. Many studies have been conducted to find an alternative fixative to update or modify formalin usage. One of them is ethanol-glycerin (EG), which suggests promising results. Despite that fact, there has yet to be any research comparing the decomposition rate between EG and formalin. This study is conducted to compare the rate of decomposition between the two fixative solutions, EG and 4% formalin on the hind limb of mice. The mice were first preserved using a standard primary fixative solution which is 10% formalin, following that procedure is preservation using advanced fixative solution, EG or 4% formalin. Upon completing the preservation steps, the mice were buried for 6 weeks and observed weekly. The stages of decomposition were assessed semi-quantitatively depending on its appearance. The hind limbs of mice that were fixed with EG solution managed to reach the last stage of decomposition, dry & remains, while the 4% formalin group of mice still remained in the previous stage, advanced decay. It is concluded that the mice hind limbs that have been previously preserved with EG advanced fixative solution has a faster decomposition rate compared to 4% formalin.
Go to : 
Anatomy learning methods using cadavers in medical science are still very much needed [1]. The development of anatomy learning methods is very diverse, including the use of technology that can be accessed easily; however, the benefits provided in direct learning with cadavers are still irreplaceable because it optimizes the use of the five senses [2]. Before the use of cadaver as an educational material, it needs to first undergo the preservation process. The main ingredient currently used in preservation is the gold standard which is 10% formaldehyde fixative [3]. However, the Department of Anatomy has developed the use of 4% formaldehyde. Apart from being safer, it has also been proven in previous studies to have a faster decomposition rate on cadavers after burial [4]. Formaldehyde or formalin has many benefits in cadaver preservation. The function of formaldehyde as a fixative solution aims to maintain cadaver integrity by preventing decomposition. Formalin, has the ability to form cross-links between proteins so as to prevent the process. Apart from optimal results, formalin is an accessible and economical material [5].
Although formaldehyde has many benefits in preserving cadaver, its usage can result in harmful effects on the health of the human body [6]. Low doses of acute exposure can cause burning sensation in nose, eyes and skin while at higher doses it can cause irritation of mucous membranes to the lower airways, causing bronchitis, pneumonia, and pulmonary edema. International Agency for Research on Cancer classifies formaldehyde as a carcinogenic substance [7]. Several studies have shown a link between long-term formaldehyde exposure and the incidence of leukemia and brain cancer [6].
Besides having a negative impact on health, the use of formalin as a cadaver preservative is also bad for the environment, for example it causes groundwater pollution. When the cadaver is buried, formaldehyde will break down [7] and be oxidized to formic acid and carbon monoxide which is then dissolved in groundwater. This in turn can lead to the phenomenon of acid rain [8, 9] Not only that, the use of formalin will also slow down the decomposition process [5], which can change the soil composition and adversely affect the surrounding vegetation [9].
Many attempts have been made to find other safer preservatives to replace the role of formaldehyde. One of the fixative fluid that is being most studied is ethanol-glycerin (EG) combination [5, 10, 11]. The combination of the two ingredients works as a good preservative by reversibly denaturing protein; thus, preventing the autolysis process as well as acting as an anti-infective agent against bacteria and other microbes. This has also been proven through the research done by Hammer et al. [10] EG can replace formalin as a fixative solution in the preservation process.
It is important for preserved tissue to be able to decompose as well or as close as the natural decomposition process (without preservatives), to prevent environmental pollution. However, there is still no research on the effect of EG fixative solution on decomposition rate after burial. Decomposition is the process of breaking down organic substances, from complex forms into simpler forms. This process is important for returning nutrients from living organisms back to the earth. Without decomposition process, the trapped nutrients in the body cannot be released, resulting in low nutrient and poor quality soil for plant growth [12].
The use of formaldehyde preservatives has the potential to cause many negative effects, both for the environment and the human body. Therefore, this study intends to find an alternative material to reduce the use of formaldehyde. One of the objectives of studying anatomy with cadavers is to see the relationship between organs and their locations [2]. One example is in musculoskeletal modelling. Most studies of human muscle architecture uses the entire leg, the part of the leg between 2 joints, or 1 joint as the specimen. For this reason, this study uses the entire hind limb of mice, which includes the structures starting from the base of the femur head to the tip of the toes in order to follow the general anatomy specimen [13], and the body as a whole. Using the whole carcass will be beneficial in reflecting the decomposition process that occurs inside the organism and not only in the muscle tissue. Mus musculus was chosen as the model of human cadaver due to its similarities in multiple organ systems, such as the nervous, musculoskeletal, gastrointestinal and cardiovascular system [14, 15] . A study comparing the microbial activity between humans and mice revealed that mice and humans share many similar types of bacteria due to their similar gastrointestinal tract and diet (omnivores). Despite the differences in genetics and physiology, this allows them to respond to decomposition similarly [15]. This study aims to compare the decomposition rate of the hind limbs of mice preserved with EG and 4% formalin advanced fixative solution.
Go to : 
This research was approved by the FMUI Ethics Committee with the letter number 828/UN2.F1/ETIK/2017. It was then extended in 2019 with letter number ND- 1390/UN2.F1/ETIK/PPM.00.02/2019.
This research was conducted at Department of Anatomy, Faculty of Medicine, University of Indonesia, and it took place from August 2019 through April 2020.
The target population is all preserved hind limbs of mice. Reachable population is all 18 pairs of Mus musculus hind limbs preserved in the Department of Anatomy, FMUI. The inclusion criteria used were samples extracted from adult male mice aged 3-6 months with body mass ranging from 20-30 g [16]. Exclusion criteria were samples with abnormalities in the limbs, especially in the hind limbs.
The sample used was the hind limbs of Mus musculus. The formula to calculate the total minimum samples for this research design is still underdeveloped. Therefore, the number of samples was calculated based on the formula used in the Data and Information Center of Indonesian Ministry of Health, which is the Federer formula [15]. With the presence of three treatment groups, the minimum sample obtained is more than or equal to 8.5 mice. The value is rounded to 9 for convenience. The following is the calculation:
Note: r=number of replications (sample size); t=number of treatment groups.
The total number of samples obtained above, is re-entered into the Mead formula, and the value must range from 10–20 for the data to be significant. It was found that 9 mice did not produce a significant number. Therefore, the value of E was set to be 15 in order to obtain a more significant result. Here is the calculation:
Note: (E=degree of freedom; N=number of samples used in the study; T=number of treatment groups).
From this calculation, the value of N=18 was obtained. Hence, the number of mice used was 18. This is in accordance with the provisions of FMUI Ethics Committee regarding the use of mice in each experimental group which is as many as 4 to 6 mice. This study uses 18 mice in total, among them are 6 mice that were not treated with preservatives (control group), 6 mice that were treated with formaldehyde fixative, and the remaining 6 mice that were treated with EG fixative.
Mice were anesthetized by injecting 100 mg/kg ketamine and 10 mg/kg xylazine. After sedation, the mice were connected to the continuous pump tube. With surgical instruments, the chest of the mouse was dissected, exposing the heart. An incision was made in the front wall of the right atrium, causing blood to flow. The 0.9% NaCl solution was immediately flowed, where it continued to flow inside the mice’s systemic tract until all blood were replaced. Through the apex of the heart, the syringe needle was inserted into the left ventricle. Upon completion, the mice were then immersed in primary fixative fluid, namely 10% formaldehyde, with a volume of 10 times of the mice. After one week, they were taken out from the 10% formaldehyde solution and rinsed with water to wash off the excess solution. Following that, they were then re-immersed in either 95% EG or 4% formalin advanced fixative solution at 4°C for another week.
After the preservation process is complete, the mice were buried for 1.5 months in standardized soil approximately 25 cm deep. The humidity of soil in the location of burial ranges from 80% to 90% with fluctuations of temperature that ranges from 25°C to 27°C, which is representative of the natural conditions in Indonesia with a tropical climate. The mice were also wrapped in a shroud upon burial to ease excavation. Observations were carried out every week where the mice were re-excavated to assess the decomposition stage of mice hind limb. The decomposition stages are assessed according to the color, texture, odor, presence of visible decomposers, such as fungi and maggots, and any other defining characteristic of a certain stage. There are 5 stages of decomposition, the operational definition are as follows [18, 19]:
At this stage, not many changes can be observed. Slight changes that may be visible are minimal greenish discoloration in the abdominal area, caused by sulfhemoglobin produced by intestinal bacteria invading the tissues after death. In addition, livor due to gravity, cracked skin, and tache noir may be observed. Autolysis begins immediately at the fresh stage, algor mortis, livor mortis, and rigor mortis are clearly visible. Insects begin to appear near the openings of the corpse, especially flies from the family Sarcophagidae and Calliphoridae. The presence of putrid odor has not yet been detected.
The onset of putrefaction begins at this stage. The discoloration is clearly visible, unlike the fresh stage. The corpse appears to expand like a balloon due to the accumulation of metabolic gases produced from anaerobic bacteria activity in various locations of the body. The increase in body pressure causes fluid to leak out of the body’s openings, giving rise to an ammoniac scent. Bodily fluids seep into the soil, converting the soil pH to alkaline, leading to an invasion of other decomposing organisms. A defining characteristic of the bloated stage is the presence of degloving or skin slippage. Few maggots may be observed invading the tissues.
Decomposition is accelerated during this stage, marked by the destruction of the outer layer of the skin due to a combination of maggot activity and bacterial putrefaction which causes the gas and fluid to seep out, deflating the body back to its original size. Hair loss and black discoloration of the ruptured skin are clearly visible. The stinging smell of ammonia is even more pronounced and the amount of decomposing organisms increase. By the end of this stage, most of the corpse’s mass have been eaten away by the maggots.
At this stage, the remaining body parts are skin, cartilage, and skull. The black discoloration of the corpse will become more even in various locations of the body, Maggots will appear less visible while the putrid odor has disappeared. The body will begin to dry out and give bones a clean polished appearance. The decomposition process decelerates during this stage.
This stage is characterized by mostly bones and hair remaining, while little amount of remaining tissues are dry and flakey. The rate of decomposition significantly decreases at this stage. It takes years for bones to be crushed completely.
After categorizing the decomposition stages, it was then converted into numerical scores presented in Table 1.
The decomposition stages data was analyzed using IBM SPSS Statistics for Windows, Version 24.0 (IBM Co., Armonk, NY, USA) with nonparametric Mann-Whitney U-test.
Go to : 
Observation of the decomposition rate of the mice’s hind limbs and body was carried out every week for 6 weeks by assessing the overall decomposition stage of each variable group. The body and hind limbs of mice did not experience significant differences in terms of decomposition stage from one another which is why they were observed as a whole. Slight differences that were found still reflected the same decomposition stage. Since the first week, the 4% formalin group and EG group of mice had experienced different stages of decomposition. Apart from the significant difference in size reduction, morphologically, the mice preserved with EG nor 4% formalin still retained the initial tissue conditions, in terms of color, shape, and size (Table 2). The difference in the stage of decomposition was more pronounced in the third week when the mice of EG group experienced a blackish color change in the abdomen, indicating active decomposition, while the mice of 4% formalin group did not experience the discoloration. In determining the stages of decomposition, the operational definition becomes the standard of judgment. Based on qualitative observations, each decomposition stage is is given a numerical score from 0–4 where 0=fresh, 1=bloated, 2=active decay, 3=advanced decay, and 4=dry, remains/skeletal.
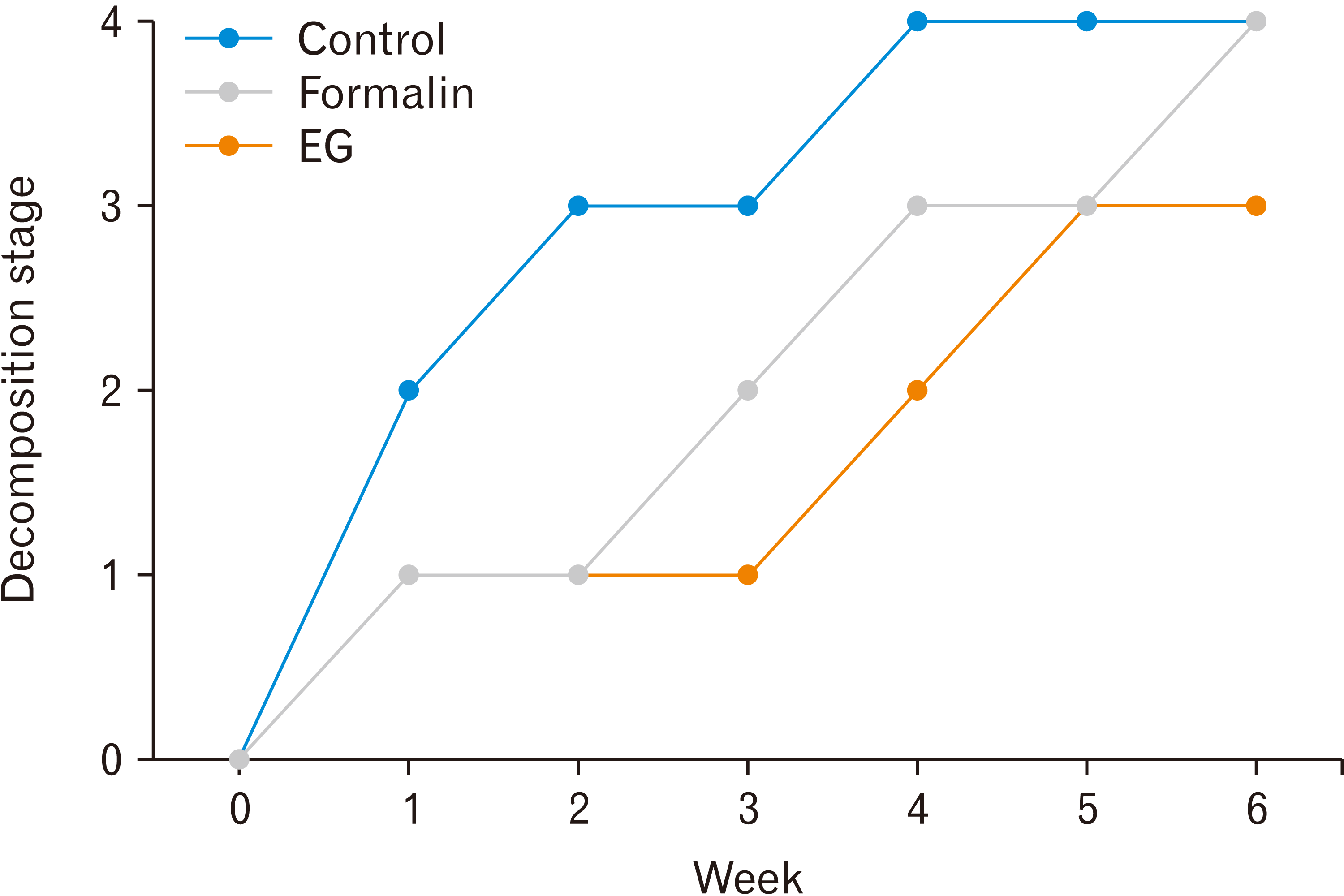
From the observations, the data were processed into graphs to see the increase of the decomposition stages per week for each group. In the last week, two groups, namely control and EG group, were classified in the dry, remains/skeletal decomposition stage, indicating the final stage of decomposition, while the 4% formalin group was still in the advanced decay stage with 4 mice being in the late advanced decay stage (Fig. 1).
Go to : 
Since ancient times, formalin fixative solution has been the gold standard used for cadaver preservation. Although it acts as an excellent preservative, formalin has numerous negative side effects for health and especially the environment. Besides being able to pollute groundwater, formalin slows down the cadaver decomposition process after burial. This will change the composition of the soil which can adversely effect the surrounding vegetation. Therefore, various attempts have been made to find an alternative fixative solution that can replace formalin, one of them is EG. The use of EG is expected to reduce the negative impact of formaldehyde, especially in increasing the cadaver decomposition process. Furthermore, this mixture is not only for safety purposes, but also to improve other weaknesses of formaldehyde, namely stiffness and increase its anti-fungal ability [20].
Based on the results of the study, there was a significant difference in the stage of decomposition between the 4% formalin and EG groups. Observed from the fresh stage in week-0, before burial of the mice, the texture of the formalin group is more rigid and stiff compared to the EG group, although visually there were no difference. In the first and second weeks, both groups still retained the initial tissue appearance. This is due to the preservative effect that is still present in mice cadavers. Signs of decomposition can only be seen from the decrease in size and skin slippage. Besides size and appearance, other parameters used are presence of putrid odor and the presence of decomposing organisms. In addition, decomposition signs can be followed by mass loss of the hind limb [21]. The 4% formalin group started to give out a mild putrid odor during the bloated stage at week 3, while the EG group started in the second week. As a comparison, ever since the first week, the control group experienced massive discoloration, and gave out a strong putrid odor, indicating the active decay stage [18].
The most significant difference in the decomposition stages between the formalin and EG groups occurred in the third week. The mice in EG group was at the active decay stage. This stage of decomposition is characterized by accelerated decay when the tissue loss is greatest, black discoloration of damaged tissue, and the presence of decomposing organisms. In contrast, the mice in formalin group were still in the bloated stage. In this study, prominent bloating did not seem to develop, but there were other signs of this stage such as mild putrid odor and skin degloving [18]. In addition, external appearance such as tissue integrity and color are still well maintained.
At week-4, mice in EG group has entered advanced decay decomposition stage. This is shown by the deceleration of decomposition such as minimal smell, if any, and the lack of decomposing organisms. Meanwhile, mice in the formalin group, previously from the bloated stage just entered active decay stage, indicated by the presence of putrid odor and maggots. After 6 weeks, the mice in EG group had entered the dry and remains decomposition stage. At this stage, the body of the mice felt very brittle and dry, while the limbs were only shreds of dry muscle and bone. The mice in formalin group only reached the late advanced decay stage. The stage was characterized by the loss of most tissue, decomposing organisms, and pungent odor [19] .The result of mass measurement and mice observation for 6 weeks provide an explanation for the differences in the rate of hind limb decomposition of mice preserved with EG and formalin. The explanation are as follows:
1. There is a difference in the fresh stage in the hind limbs of mice preserved with EG and formalin. The formalin group is more rigid than the EG group.
2. There was a different bloated stage in the hind limbs of mice preserved with EG and formalin. The EG group had a bloated stage duration that was 1 week faster and the putrid odor appeared more quickly which was during the second week, compared to the formalin group that survived the bloated stage for 2 weeks and the pungent odor appeared at week
3. There is a difference in the active decay stage in the hind limbs of mice preserved with EG and formalin. The EG group had more prominent discolorations and decomposing organisms than the formalin group.
4. There are differences in the advanced decay stage in the hind limbs of mice preserved with EG and formalin. The EG group had more size reduction than the mice in the formalin group at the same stage.
5. The dry and remains stage on the hind limbs of mice preserved with EG and formalin could not be compared because at week 6 the formalin group was still in the late stage of the advanced decay where it was only slightly dry.
From the statistical tests and explanation above, it is concluded that there is a significant difference between the decomposition rate of the hind limbs of mice preserved with EG and formalin.
The difference in decomposition rate may be caused by how each of the two preservative solutions work. Formalin prevents the decomposition process by forming cross-links between proteins through the insertion of methylene groups between nitrogen bonds. The formation of this covalent bond provides more durable tissue fixation effect [4]. In contrast to EG, this organic solution works by stopping cell metabolism and autolysis through the process of protein denaturation, especially the hydrolytic enzymes released by cells after death, in a reversible manner [10, 18]. Denatured proteins may return to their initial structure once the concentration of EG within the mice is reduced over time. The reversibility effect due to the decrease in the EG concentration makes decomposition easier to occur compared to the 4% formalin group.
The benefit of this research is that it can be used as reference for future research regarding alternatives for advanced fixative solution of formalin, especially solutions that are EG-based. Moreover, in contrast to existing research on the fixative ability of EG tissue, this study examines another effect of EG fixative solution that is the effect on tissue decomposition after burial.
In conclusion, there was a difference in decomposition rate in the hind limbs of mice preserved with 4% advanced fixative formalin and EG. The hind limbs of mice fixed with EG solution had a faster decomposition rate after burial compared to 4% formalin.
Previous studies on cadaver preservation generally uses formaldehyde as a preservative. However, the use of formaldehyde preservative has the potential to cause many negative effects, both for the environment and the body. Therefore, this study intends to find an alternative material to reduce the use of formalin, which is EG solution.
Go to : 
Acknowledgements
We would like to thank Universitas Indonesia Research Grant, International Indexed Publication (PUTI) program 2020 with contract number NKB- 913/UN2.RST/HKP.05.00/2020 for funding this research. This article was presented in the 5th International Conference and Exhibition on Indonesian Medical Education and Research Institute (5th ICE on IMERI), Faculty of Medicine, Universitas Indonesia. We thank the 5th ICE on IMERI committee, who have supported the peer-review and manuscript preparation before submitting it to the journal.
Go to : 
Notes
Author Contributions
Conceptualization: RM. Data acquisition: RM. Data analysis or interpretation: ANW, RM. Drafting of the manuscript: ANW, RM, SK, DF. Critical revision of the manuscript: RM. Approval of the final version of the manuscript: all authors.
Go to : 
References
1. Inthasan C, Vaseenon T, Mahakkanukrauh P. 2020; Anatomical study and branching point of neurovascular structures at the medial side of the ankle. Anat Cell Biol. 53:422–34. DOI: 10.5115/acb.20.087. PMID: 32814704. PMCID: PMC7769108.

2. Dissabandara LO, Nirthanan SN, Khoo TK, Tedman R. 2015; Role of cadaveric dissections in modern medical curricula: a study on student perceptions. Anat Cell Biol. 48:205–12. DOI: 10.5115/acb.2015.48.3.205. PMID: 26417481. PMCID: PMC4582164.

3. Thavarajah R, Mudimbaimannar VK, Elizabeth J, Rao UK, Ranganathan K. 2012; Chemical and physical basics of routine formaldehyde fixation. J Oral Maxillofac Pathol. 16:400–5. DOI: 10.4103/0973-029X.102496. PMID: 23248474. PMCID: PMC3519217.

4. Yogawisea S. 2017; Pengaruh konsentrasi cairan fiksatif formalin 10% dan 4% pada proses dekomposisi otot tungkai belkanag mencit.
5. Brenner E. 2014; Human body preservation- old and new techniques. J Anat. 224:316–44. DOI: 10.1111/joa.12160. PMID: 24438435. PMCID: PMC3931544.
6. Swenberg JA, Moeller BC, Lu K, Rager JE, Fry RC, Starr TB. 2013; Formaldehyde carcinogenicity research: 30 years and counting for mode of action, epidemiology, and cancer risk assessment. Toxicol Pathol. 41:181–9. DOI: 10.1177/0192623312466459. PMID: 23160431. PMCID: PMC3893912.
7. Agency for Toxic Substances and Disease Registry. 2021. Public health statement for formaldehyde [Internet]. Agency for Toxic Substances and Disease Registry;Chamblee, GA: Available from: https://www.atsdr.cdc.gov/phs/phs.asp?id=218&tid=39. cited 2018 Sep 15.
8. Saraswat P, Nirwan P, Saraswat S, Mathur P. 2008; Biodegradation of dead bodies including human cadavers and their safe disposal with reference to mortuary practice. J Indian Acad Forensic Med. 30:273–80.
9. Żychowski J, Bryndal T. 2015; Impact of cemeteries on groundwater contamination by bacteria and viruses- a review. J Water Health. 13:285–301. DOI: 10.2166/wh.2014.119. PMID: 26042963.
10. Hammer N, Löffler S, Feja C, Sandrock M, Schmidt W, Bechmann I, Steinke H. 2012; Ethanol-glycerin fixation with thymol conservation: a potential alternative to formaldehyde and phenol embalming. Anat Sci Educ. 5:225–33. DOI: 10.1002/ase.1270. PMID: 22434588.

11. Hammer N, Löffler S, Bechmann I, Steinke H, Hädrich C, Feja C. 2015; Comparison of modified Thiel embalming and ethanol-glycerin fixation in an anatomy environment: potentials and limitations of two complementary techniques. Anat Sci Educ. 8:74–85. DOI: 10.1002/ase.1450. PMID: 24706536.

12. Cockle DL, Bell LS. 2017; The environmental variables that impact human decomposition in terrestrially exposed contexts within Canada. Sci Justice. 57:107–17. DOI: 10.1016/j.scijus.2016.11.001. PMID: 28284436.

13. Ruggiero M, Cless D, Infantolino B. 2016; Upper and lower limb muscle architecture of a 104 year-old cadaver. PLoS One. 11:e0162963. DOI: 10.1371/journal.pone.0162963. PMID: 28033339. PMCID: PMC5199092.

14. Rosenthal N, Brown S. 2007; The mouse ascending: perspectives for human-disease models. Nat Cell Biol. 9:993–9. DOI: 10.1038/ncb437. PMID: 17762889.

15. Nguyen TL, Vieira-Silva S, Liston A, Raes J. 2015; How informative is the mouse for human gut microbiota research? Dis Model Mech. 8:1–16. DOI: 10.1242/dmm.017400. PMID: 25561744. PMCID: PMC4283646.

16. Johns Hopkins University. 2019. Animal care and use committee. The mouse [Internet]. Johns Hopkins University;Baltimore, MD: Available from: http://web.jhu.edu/animalcare/procedures/mouse.html. cited 2019 Jul 23.
17. Banerjee A, Chaudhury S. 2010; Statistics without tears: populations and samples. Ind Psychiatry J. 19:60–5. DOI: 10.4103/0972-6748.77642. PMID: 21694795. PMCID: PMC3105563.

18. Almulhim AM, Menezes RG. 2020. Evaluation of postmortem changes [Internet]. StatPearls Publishing;Treasure Island, FL: Available from: https://www.ncbi.nlm.nih.gov/books/NBK554464/. cited 2019 Jul 15.
19. Lee Goff M. 2009; Early post-mortem changes and stages of decomposition in exposed cadavers. Exp Appl Acarol. 49:21–36. DOI: 10.1007/s10493-009-9284-9. PMID: 19554461.

20. Tamayo-Arango L, Garzón-Alzate A. 2018; Preservation of animal cadavers with a formaldehyde-free solution for gross anatomy. J Morphol Sci. 35:136–41. DOI: 10.1055/s-0038-1669434.

21. Margiana R, Wijaya AN, Kusumaningtyas S, Furqonita D, Rahmadini . 2021; The comparison of hind limb mass loss in house mice preserved with advanced fixative solutions of ethanol- glycerin and 4% formaldehyde. Ann RSCB. 25:2087–93.
Go to : 




 PDF
PDF Citation
Citation Print
Print





















 XML Download
XML Download