Discussion
The digastric muscle, as indicated by the very name, possesses two muscle bellies [
11-
13], and is presented with that denomination in several species.
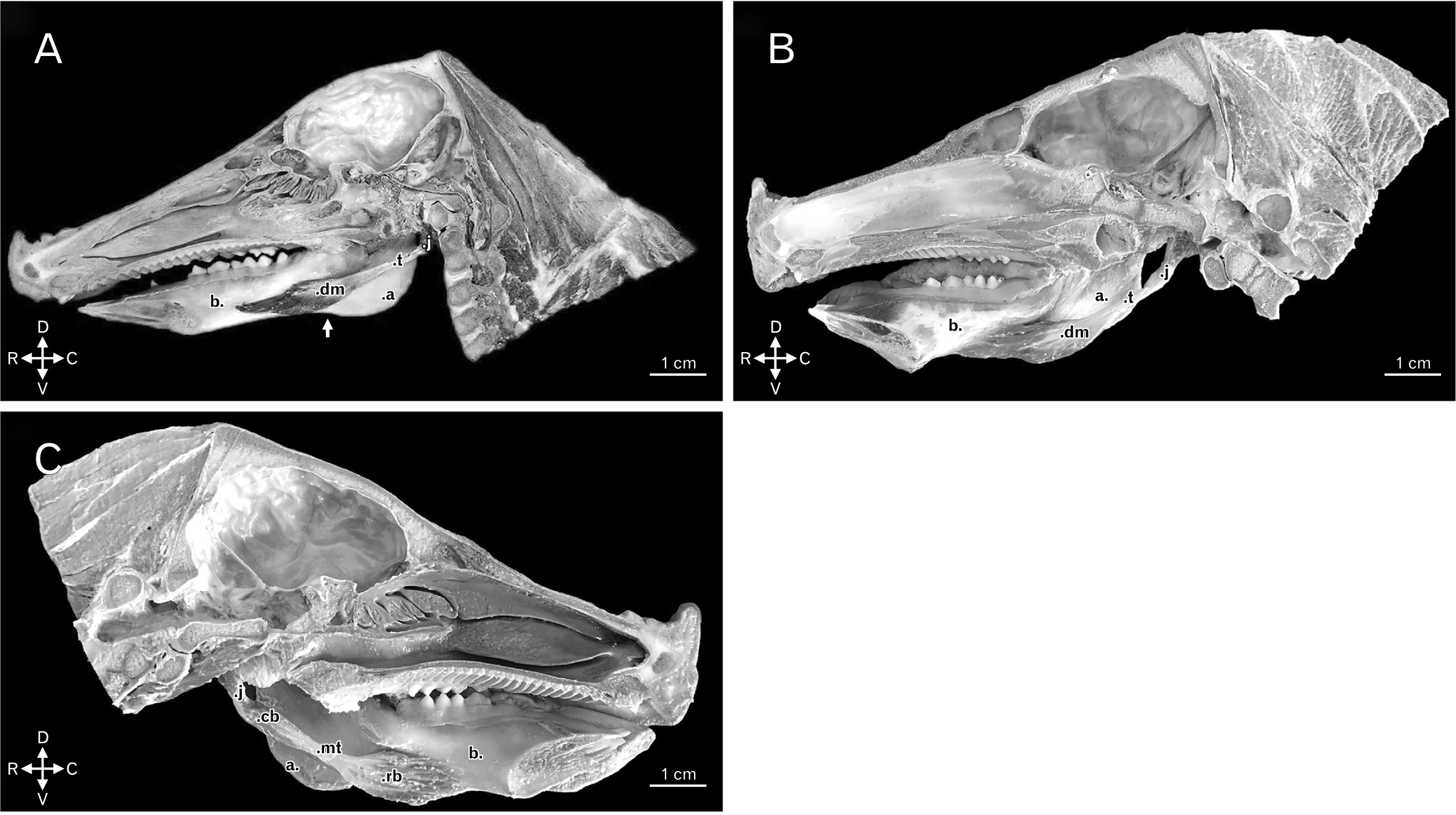
In regard of the your origin, in boars and domestic swine alike, it has taken place in a tendinous fashion departed from the jugular process of the occipital bone, just as described by Getty [
1] for ruminants and swine. In equines and domestic carnivores that have taken place directly through its muscle fibers, also attached in the jugular process of the occipital bone [
1,
14,
15].
Upon analyzing the origin of the digastric muscle in a number of wild animals, it was noticed that the same had also taken place in the jugular process of the occipital bone for the crab-eating raccoon (
Procyon cancrivorus) [
16], the black-eared opossum (
Didelphis marsupialis), the moonrat (
Echinosorex gymnurus), the white-tailed deer (
Odocoileus virginianus), the fox squirrel (
Sciurus niger), and the brown rat (
Rattus norvegicus) [
17]. However, for the North American river otter (
Lutra canadensis), the sea otter (
Enhydra lutris), the striped skunk (
Mephitis mephitis), the North American badger (
Taxidea taxus), the american pine marten (
Martes americana), the wolverine (
Gulo luscus), and the american mink (
Mustela vison), apart from the structure quoted, the said muscle would also originated from the jugulomastoid crist [
18]. Yet, for the caspian seal (
Phoca caspica), the reported region was the ventral surface of the auditory bulla of the occipital bone [
19], whilst, for the kangaroo (
Macropus robustus), the paraoccipital process of the occipital bone was reported [
20].
Spataru et al. [
21] whilst studying the red squirrel (
Sciurus vulgaris), and Ferreira et al. [
22] whilst analyzing the black-capped capuchin (
Cebus apella), have pointed out that the digastric muscle would originate in the paracondylar process of the occipital bone however, in the first species mentioned the origin would also involve the caudoventral region of the tympanic bulla. Yet, in rabbits, the anatomic region quoted by Muhl and Newton [
23] has been the paramastoid process at the base of skull. In humans, Madeira [
12], Standring [
24], and Khan and Bordoni [
13] would claim the mastoid incisure of the temporal bone as being the point of muscle origin.
In the domestic ruminants [
1,
11], equines [
1,
15,
25], crab-eating raccoon [
16], red squirrel [
21], black-eared opossum, moonrat, white-tailed deer, fox squirrel, and brown rat [
17], the digastric muscle would pursue its path from its caudal belly, by means of a strong intermediate tendon, to have its continuity rostrally interrupted by the emergence of the rostral belly of the digastric muscle. Peculiarly in equines, the caudal belly would present the occipitomandibular part set laterally and inserted in the caudal edge of the angle of the mandible [
1,
15,
25,
26].
Yet, in the domestic carnivores, only one tendinous intersection [
1,
27], small and hardly noticeable, would represent the intermediate tendon and indicate the digastric nature of the muscle [
14]. For the mustelidae studied by Scapino [
18], quite similar a description to that had been given, whilst, in the caspian seal the caudal belly would insert in the ventral region of the rostral belly of the digastric muscle itself [
19].
Save the different points of origin of the digastric muscle, the description made by Ferreira et al. [
22] for the black-capped capuchin bore great resemblance to that which had been reported by Madeira [
12], Moore et al. [
28], Standring [
24], O’Daniel [
29], Al-Missri and Khalili [
30], and Khan and Bordoni [
13] in humans. In such instances, the rostral and caudal bellies, anterior and posterior in humans, would also be united by an intermediate tendon in common, albeit attached to the hyoid bone by fibers of the fasciae from the cervical region.
In rabbits and kangaroos, only one muscle belly would respectively be observed by Muhl and Newton [
23] and Tomo et al. [
20]. In the domestic species, the digastric muscle, after its tendo-aponeurotic origin, would stretch unhindered to insert with its muscle fibers in the medial surface of the mandible [
23]. Yet, in the wild species, that muscle would be comprised by a fascicle, rounded and small in its origin, but wider, thick, and tendinous in its insertion [
20].
For the domestic swine, Getty [
1] referenced such muscle just as the occipitomandibular part of the digastric muscle, whilst Kyllar et al. [
7], in mentioning a muscular composition of two continuous bellies would use the denomination of digastric muscle. Before the indetermination, and now supported by the representations and descriptions made herein for boars and domestic swine, it is known that the muscle belly present is single, and whilst corroborating with Getty [
1] and Kyllar et al. [
7] in regard of its insertion in the midventral edge of the body of the mandible, it is assumed that the similarities between those species are highly relevant.
It has been worth to mention observing an anatomic variation in regard of the number of muscle bellies present in a female swine specimen, which characterized one muscle with two muscle bellies individualized by a middle tendon. In humans, morphological variations of the digastric muscle are common [
31] and usually related to supranumeric bellies [
32-
34], and to differences in the form and site of insertion [
35-
37]. This reporting is always crucial, since those variations may interfere with the interpretation of imagery scans [
35] and in the clinical and surgical procedures of the region [
38].
Now, in respect of the insertion of the digastric muscle through its rostral belly, in accordance with Getty [
1], Singh [
26], and König and Liebich [
15] for equines, and with Turnbull [
17] for the black-eared opossum, this muscle has taken place through the aforementioned anatomic point for the two specimens of suidae. In the domestic ruminants it has been observed in the medial [
1,
15] and ventral edges of the body of the mandible [
11]. For the domestic carnivores, Getty [
1] and Budras et al. [
14] have reported that said attachment would be visualized in the ventral edge of the mandible, whilst König and Liebich [
15] claimed the insertion on the ventromedial surface of that osseous structure, opposed to the lower canine tooth. Still for the domestic carnivores, Evans and DeLahunta [
27] has indicated only the region of the body of the mandible as the area of insertion just as Pereira et al. [
16] did whilst studying specimens of crab-eating raccoon. In mustelids, the reported regions would be the ventral, lateral, and medial edges of the body of the mandible, ventralwise to the first or the second lower molars until nearly the level of the mandibular foramen [
18].
Spataru et al. [
21] described that, in the red squirrel, the attachment of the rostral belly of the digastric muscle would take place together with the mylohyoid and geniohyoid muscles upon the merger of the same in the intermandibular space. Yet, in the moonrat, the muscle insertion has been visualized on the ventrolateral surface of the mandible, ventralwise to the lower molars. Nevertheless, for the white-tailed deer, the insertion would mostly take place in the ventromedial area of the mandible, rostralwise to the attachment of the medial pterygoid muscle and, in a lesser scale, medialwise in the ramus of the mandible. In the fox squirrel and the brown rat, the ventromedial edge of the rostral third of the body of the mandible has been verified as the site of muscle insertion [
17]. In the caspian seal the same would take place on the medial and ventromedial surfaces of the body of the mandible after a first attachment in the angular process of the mandible [
19].
Ferreira et al. [
22] reported in the black-capped capuchin, the rostral belly would insert in the body of the mandible, whilst stretching out from its incisive ventral edge as far as the ventral edge of the molar portion of the bone. In humans the attachment would take place through the anterior belly in the digastric fossa of the mandible [
13,
24,
29,
30].
For the identification of the possible action of a given muscle and the consequent motion executed in the body of the mandible, we may quote that the topographical and directional observation of the fibers is of the utmost importance. As from that analysis in the boars and in the domestic swine it has been possible to suggest that the digastric muscle would perform in the lowering and in the retracting of the mandible, thus partially corroborating the information recorded in the better part of the literature on domestic animals [
1,
14,
15,
23,
26,
27,
39,
40], on wild animals [
16,
18,
19], and on humans [
13,
24,
29], wherein reports on that latter action are mostly lacking in the descriptions pertinent to the digastric muscle.
Nevertheless, Madeira [
12] have claimed that, in human kind, upon it is sliding by the loop that attaches it to the hyoid bone, said muscle contracts as a whole and drags the mandible backwards, thus contributing for the lowering of the same in synergy with the lateral pterygoid muscle. In wild animals, motions of backward dragging associated with the amplification of the temporomandibular articulation have been described only for the red squirrel [
21]. In suidae, the ventral limit of the very long paroccipital process extends far below the palate and reaches the ramus of the mandible, which causes the digastric muscle to exert a backward traction on the mandible [
39]. Furthermore, the functions of elevation of the hyoid bone over deglutition [
29,
41] and the lateralization of the mandible during the opening of the mouth [
40] have also been mentioned.
In quite broad a manner, the muscles may be classified in regard of origin and insertion [
4,
11], of the number of muscle bellies present, of the position in the skeleton [
11], of the orientation of the fibers [
4,
11], and of the shape and function [
4]. So, just as noted by Ribeiro et al. [
42], we reaffirm the inconvenience of using the term ‘digastric’ to name the muscle under scrutiny in boars, hence only one muscle belly has been observed in that species. Ergo, it is suggested that the denomination of mandibular retractor muscle can be adopted based on the muscle functionality upon analysis of its topography, rather than on the number of muscle bellies present as has been made for the other animals. By the way, given the similarity of this muscle in the domestic swine, the use of the same criteria for that species becomes pertinent even for any other species which has morphologically and physiologically similar a muscle to this one.
Still on the guidance for anatomical denomination, it has been noticed that the Nomina Anatomica Veterinaria [
10] and the Terminologia Anatomica [
43] contradict one another in regard of the description of the ‘intermediate’ tendon of the digastric muscle, in view of the distribution of the muscle as a whole upon analyzing the specimens in anatomical position.
In veterinary science, upon our referring to the indicative terms of position and orientation of three different structures distributed along a longitudinal axis, cranial, middle, and caudal are applied should the same be located along the head, neck, and trunk (whilst altering to hands, feet, and when two or more structures of the head are regarded) [
15]. In a similar fashion, the same rules are applied for humans, albeit the terms used are anterior, middle, and posterior when the structures are observed in a sagittal axis [
44]. Therefore, the characterization of the said tendon as the middle tendon of the digastric muscle becomes valid, hence the same is located middlewise to the rostral and caudal bellies of both domestic and wild animals (longitudinal axis), and to the anterior and posterior bellies of humans (sagittal axis).
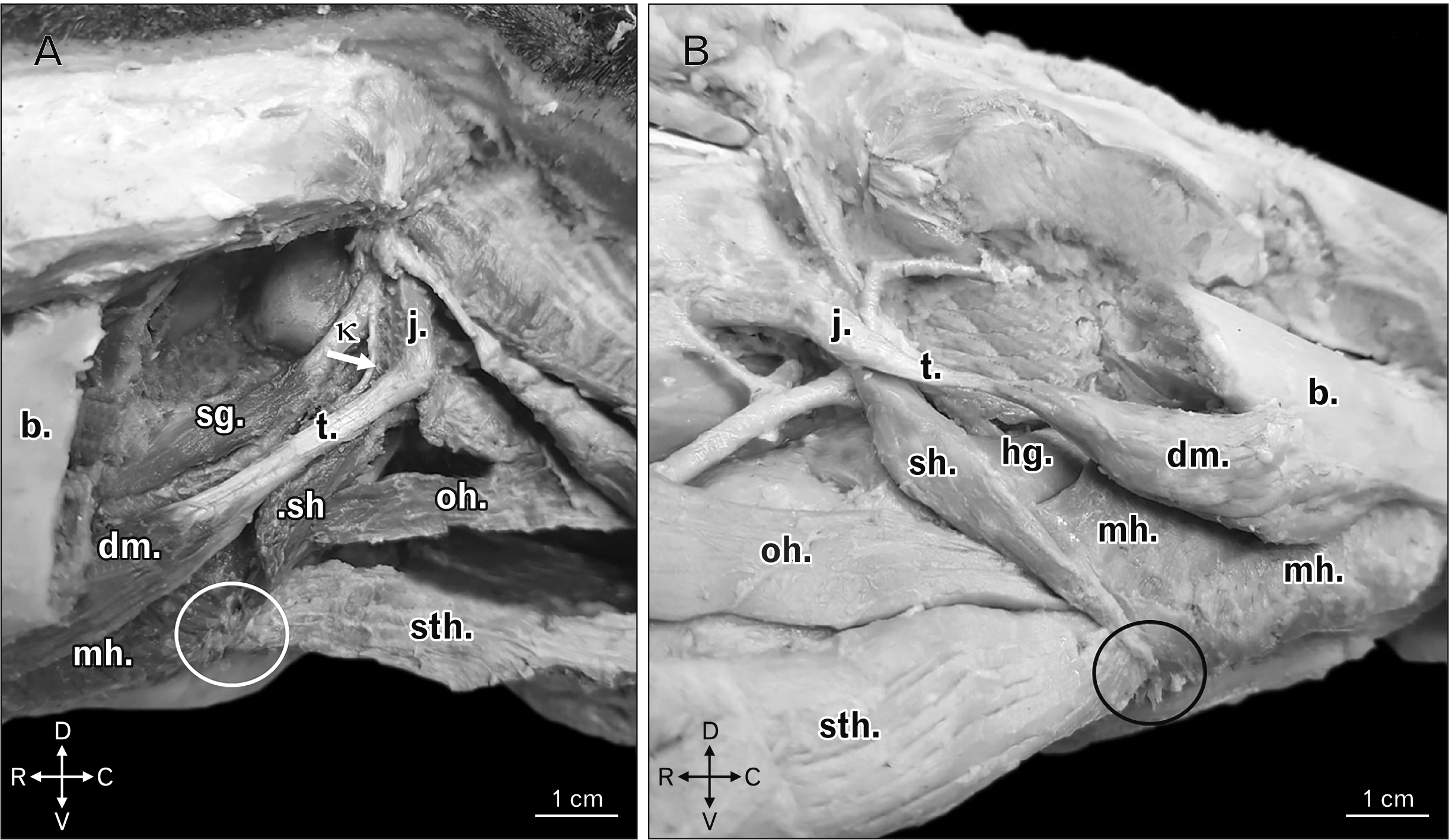
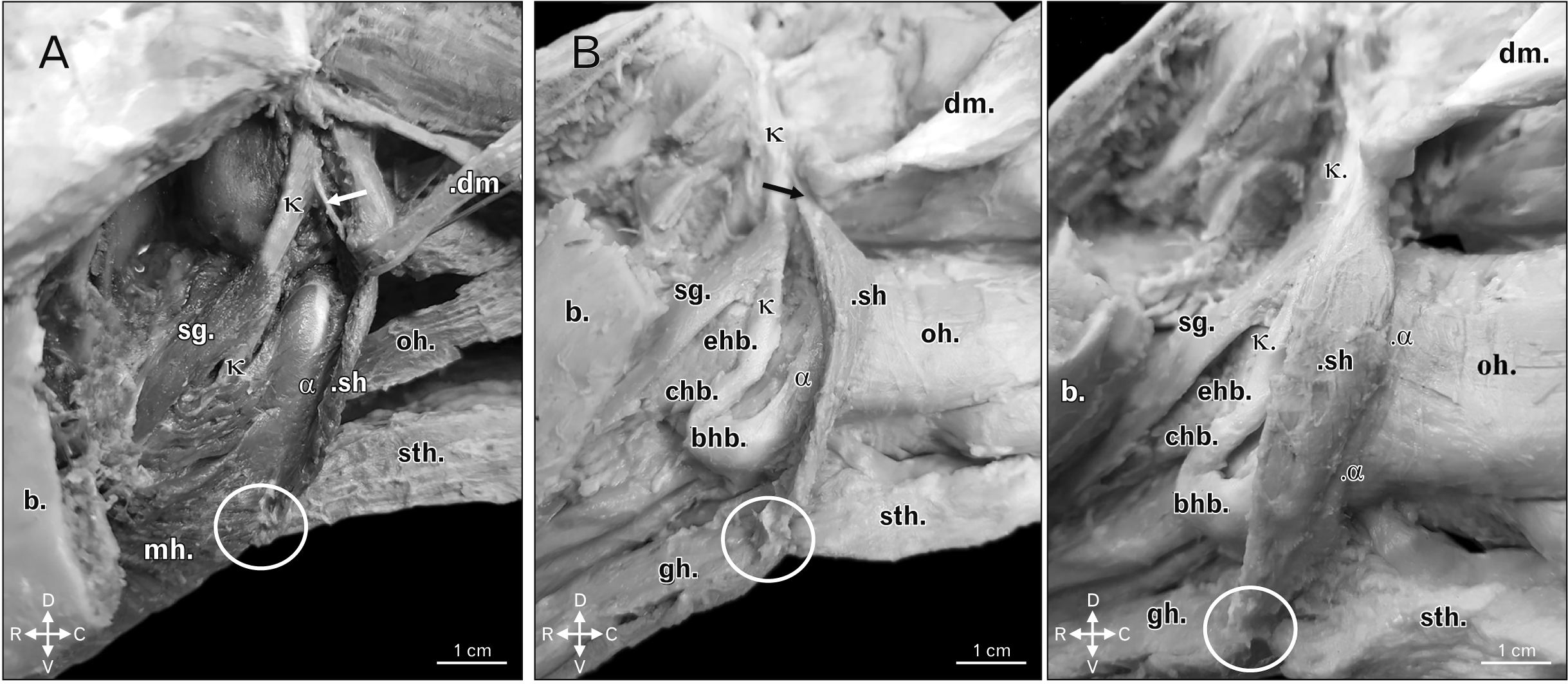
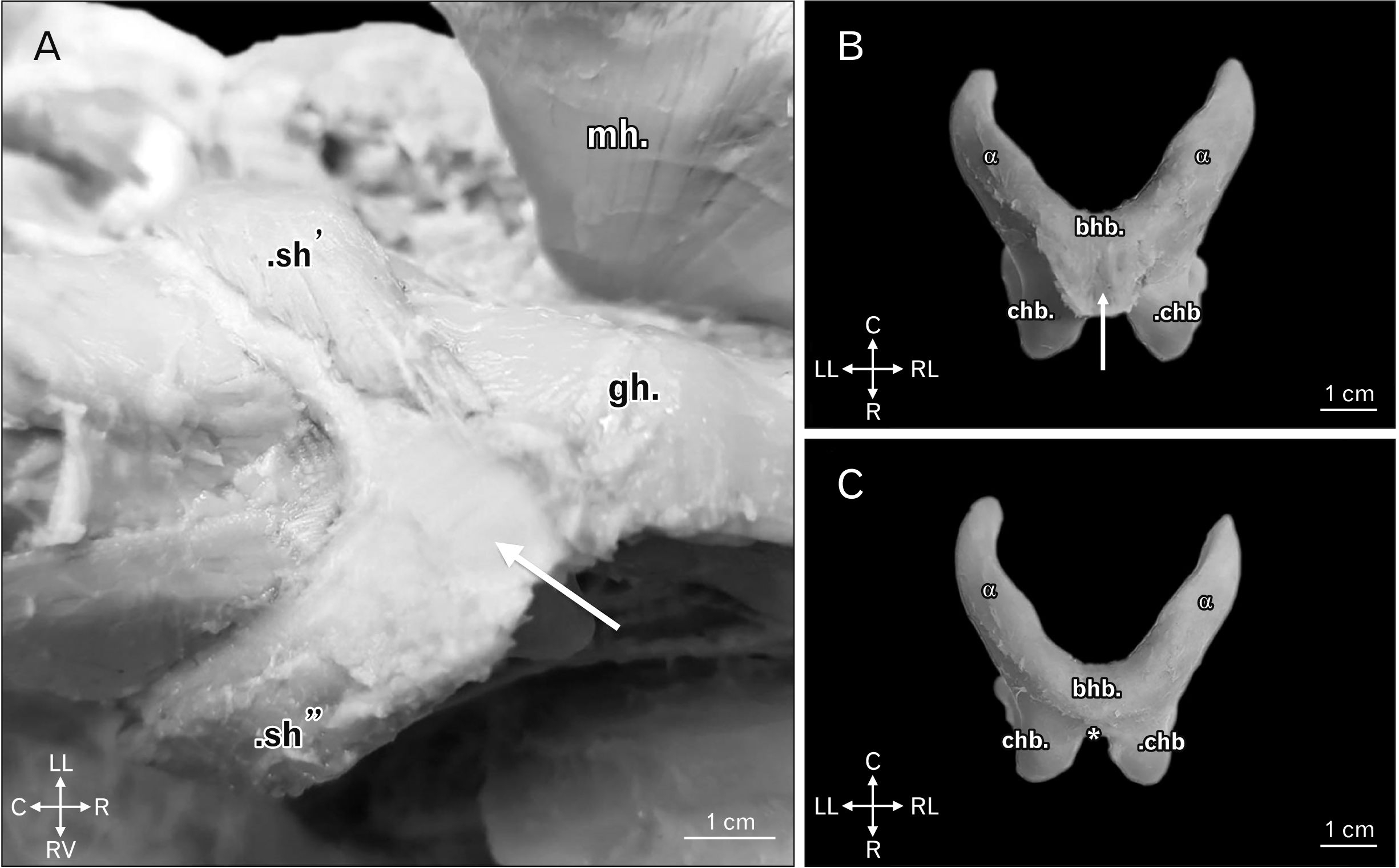
Yet, in respect of the anatomy of the stylohyoid muscle in the domestic ruminants, its origin has taken place by a long and slim tendon attached to the muscle angle of the stylohyoid bone. The insertion is muscular and occurs in the lateral end of the basihyoid bone [
1]. Now, in the domestic carnivores, König and Liebich [
15] have claimed that such muscle would originate from the temporal bone, and insert in the thyrohyoid bone. In equines, the origin has taken place in a tendinous fashion in the muscle angle of the dorsal end of the thyrohyoid bone, and upon the formation of a spindle-shaped muscle belly inserted by a tendon in the rostral portion of the same bone [
1].
In humans, this muscle is attached by a tendon to the posterior face of the stylohyoid process, next to the base of the same and, upon its having stretched as far as the body of the hyoid bone, at the junction with the greater horn, may end in the digastric or supra or, even, infra-hyoid muscles [
24,
45]. It is pointed out that, in the boars and in the domestic swine, the stylohyoid muscle originated by a long tendon in the laterocaudal edge of the dorsal third of the stylohyoid bone and inserted as a muscle belly - firstly, in the lateral and caudal edges form the mid third as far as the ventral extremity of the thyrohyoid bone; and in a secondary fashion, rostroventrally in a region of insertion of the sternohyoid, the geniohyoid, and the mylohyoid muscles, which presented a short and aponeurotic morphology, and which has laterolaterally stretched so that the counterlateral muscles of common insertion ended up by uniting bilaterally in that site at the level of a median ventral spot.
Generally, in the domestic animals the stylohyoid muscle moves the hyoid bone and the larynx in a caudodorsal manner [
15], whilst in humans its function is associated to the raising and the retracting of the hyoid bone, with the consequent stretching of the floor of the mouth [
24,
45]. Together with the other hyoid muscles, it also stabilizes the hyoid bone, aids in the opening of the mouth, in the flexing of the neck, and in the production of acute sounds [
45]. In the boars and in the domestic swine, the possible function of the muscle in question enfolds the stabilization and the positioning of the larynx, whilst also performing in synergy with the digastric muscle and as a floor of fixation and support for the hyoid bone during motion of the tongue in the process of deglutition. It is important to highlight that no relationship of contact between the hyoid bone and the digastric muscle has been observed in the suidae.
For the equines, Getty [
1], Budras et al. [
25], Singh [
26], and König and Liebich [
15] informed that the ‘intermediate’ tendon of the digastric muscle had perforated the tendon of insertion of the stylohyoid muscle, wherein the same would be endowed with a synovial sheath [
1]. Yet, in humans, the ‘intermediate’ tendon also crosses the stylohyoid muscle next to its insertion [
12,
24] although it binds, right after, to the body and the greater horn of the hyoid bone by means of a fibrous sling [
28], whereby it slides in the anterior and posterior directions [
12,
28] in a manner similar as that of a pulley [
28,
46], and at times it also presents a synovial sheath [
24].
With such grounds, we are able to infer that, respectively in equines and humans, the stylohyoid muscle and the fibrous sling also act as a muscular annex in the guise of a reflection pulley for the digastric muscle whilst providing for a retransmission of its power over mastication and facilitating the performance of their actions. For boars and domestic swine it is proposed that, even upon lack of observation of a specific muscular annex for the structures regarded in this study, the morphology of the digastric muscle permits its functions to be carried out at the requirement of less energy spent, hence the presence of a more elongated tendon of origin in lieu of a second muscle belly can create a more efficient mechanism for the storing and the release of the elastic energy created by the tensioning of that tissue which also provides for greater muscle strength, as has been suggested by Witvrouw et al. [
47]. This characteristic is potentiated by the topographical and biomechanical relationship of the digastric muscle with the tendon of origin of the stylohyoid muscle, as this one also acts physically in the guise of a lever arm for that muscle.
It is concluded that unlike the vast majority of the species presented in the literature, for boars and domestic swine the digastric muscle presented only one muscle belly as an anatomical component, of a tendinous origin in the jugular process of the occipital bone and muscle insertion in the midventral edge of the caudal two thirds of the body of the mandible. It is believed that this morphology can favor the storing and the release of elastic energy created by the tendinous tensioning whilst providing for greater muscle power at lesser energy expense, which would facilitate the breaking of harder foods. This muscle acts in the lowering and the retracting of the mandible.
For the stylohyoid muscle the tendinous origin in the laterocaudal edge of the dorsal third of the stylohyoid bone, and the muscle insertion - primarily - in the lateral and caudal edges from the mid third as far as the ventral extremity of the thyrohyoid bone, and secondarily as a laterolateral aponeurotic blade which bilaterally united a common insertion with the sternohyoid, the geniohyoid, and the mylohyoid muscles in a median ventral region have been similar for the two suidae specimens. Thus, it can be presumed that this muscle possibilitates a greater degree of stability in the positioning of the larynx whilst also performing in the guise of a lever arm in synergy with the digastric muscle, and as a floor of fixation and support for the hyoid bone during motion of the tongue upon deglutition. By comparison, the morphology observed was different from that which had been described for the other animals and humans, thus expanding the bulk of information available in regard of their functions - completely unknown for the suidae until then.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download