3. Behnisch T, Reymann KG. 1993; Co-activation of metabotropic glutamate and N-methyl-D-aspartate receptors is involved in mechanisms of long-term potentiation maintenance in rat hippocampal CA1 neurons. Neuroscience. 54:37–47. DOI:
10.1016/0306-4522(93)90381-O. PMID:
8515845.

4. Piletz JE, Aricioglu F, Cheng JT, Fairbanks CA, Gilad VH, Haenisch B, Halaris A, Hong S, Lee JE, Li J, Liu P, Molderings GJ, Rodrigues AL, Satriano J, Seong GJ, Wilcox G, Wu N, Gilad GM. 2013; Agmatine: clinical applications after 100 years in translation. Drug Discov Today. 18:880–93. DOI:
10.1016/j.drudis.2013.05.017. PMID:
23769988.

5. Raasch W, Regunathan S, Li G, Reis DJ. 1995; Agmatine, the bacterial amine, is widely distributed in mammalian tissues. Life Sci. 56:2319–30. DOI:
10.1016/0024-3205(95)00226-V. PMID:
7791519.

6. Molderings GJ, Burian M, Homann J, Nilius M, Göthert M. 1999; Potential relevance of agmatine as a virulence factor of Helicobacter pylori. Dig Dis Sci. 44:2397–404. DOI:
10.1023/A:1026662316750. PMID:
10630488.
7. Osborne DL, Seidel ER. 1990; Gastrointestinal luminal polyamines: cellular accumulation and enterohepatic circulation. Am J Physiol. 258(4 Pt 1):G576–84. DOI:
10.1152/ajpgi.1990.258.4.G576. PMID:
2333971.

8. Benamouzig R, Mahé S, Luengo C, Rautureau J, Tomé D. 1997; Fasting and postprandial polyamine concentrations in the human digestive lumen. Am J Clin Nutr. 65:766–70. DOI:
10.1093/ajcn/65.3.766. PMID:
9062527.

9. Li YF, Chen HX, Liu Y, Zhang YZ, Liu YQ, Li J. 2006; Agmatine increases proliferation of cultured hippocampal progenitor cells and hippocampal neurogenesis in chronically stressed mice. Acta Pharmacol Sin. 27:1395–400. DOI:
10.1111/j.1745-7254.2006.00429.x. PMID:
17049113.

10. Raghavan SA, Dikshit M. 2004; Vascular regulation by the L-arginine metabolites, nitric oxide and agmatine. Pharmacol Res. 49:397–414. DOI:
10.1016/j.phrs.2003.10.008. PMID:
14998549.
11. Popolo A, Adesso S, Pinto A, Autore G, Marzocco S. 2014; L-Arginine and its metabolites in kidney and cardiovascular disease. Amino Acids. 46:2271–86. DOI:
10.1007/s00726-014-1825-9. PMID:
25161088.

13. Wang CC, Chio CC, Chang CH, Kuo JR, Chang CP. 2010; Beneficial effect of agmatine on brain apoptosis, astrogliosis, and edema after rat transient cerebral ischemia. BMC Pharmacol. 10:11. DOI:
10.1186/1471-2210-10-11. PMID:
20815926. PMCID:
PMC2941483.

14. Vlassara H, Bucala R, Striker L. 1994; Pathogenic effects of advanced glycosylation: biochemical, biologic, and clinical implications for diabetes and aging. Lab Invest. 70:138–51. PMID:
8139257.
15. Li G, Regunathan S, Barrow CJ, Eshraghi J, Cooper R, Reis DJ. 1994; Agmatine: an endogenous clonidine-displacing substance in the brain. Science. 263:966–9. DOI:
10.1126/science.7906055. PMID:
7906055.

17. Moretti M, Matheus FC, de Oliveira PA, Neis VB, Ben J, Walz R, Rodrigues AL, Prediger RD. 2014; Role of agmatine in neurodegenerative diseases and epilepsy. Front Biosci (Elite Ed). 6:341–59. DOI:
10.2741/710. PMID:
24896210.

18. Watts D, Pfaffenseller B, Wollenhaupt-Aguiar B, Paul Géa L, Cardoso TA, Kapczinski F. 2019; Agmatine as a potential therapeutic intervention in bipolar depression: the preclinical landscape. Expert Opin Ther Targets. 23:327–39. DOI:
10.1080/14728222.2019.1581764. PMID:
30764678.

19. Feng Y, LeBlanc MH, Regunathan S. 2005; Agmatine reduces extracellular glutamate during pentylenetetrazole-induced seizures in rat brain: a potential mechanism for the anticonvulsive effects. Neurosci Lett. 390:129–33. DOI:
10.1016/j.neulet.2005.08.008. PMID:
16125317. PMCID:
PMC2920494.

20. Olmos G, DeGregorio-Rocasolano N, Paz Regalado M, Gasull T, Assumpció Boronat M, Trullas R, Villarroel A, Lerma J, García-Sevilla JA. 1999; Protection by imidazol(ine) drugs and agmatine of glutamate-induced neurotoxicity in cultured cerebellar granule cells through blockade of NMDA receptor. Br J Pharmacol. 127:1317–26. DOI:
10.1038/sj.bjp.0702679. PMID:
10455281. PMCID:
PMC1760666.

21. Kim JY, Lee YW, Kim JH, Lee WT, Park KA, Lee JE. 2015; Agmatine attenuates brain edema and apoptotic cell death after traumatic brain injury. J Korean Med Sci. 30:943–52. DOI:
10.3346/jkms.2015.30.7.943. PMID:
26130959. PMCID:
PMC4479950.

23. Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. 2003; Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 301:805–9. DOI:
10.1126/science.1083328. PMID:
12907793.

24. Song J, Hur BE, Bokara KK, Yang W, Cho HJ, Park KA, Lee WT, Lee KM, Lee JE. 2014; Agmatine improves cognitive dysfunction and prevents cell death in a streptozotocin-induced Alzheimer rat model. Yonsei Med J. 55:689–99. DOI:
10.3349/ymj.2014.55.3.689. PMID:
24719136. PMCID:
PMC3990080.

26. Kim JH, Yenari MA, Giffard RG, Cho SW, Park KA, Lee JE. 2004; Agmatine reduces infarct area in a mouse model of transient focal cerebral ischemia and protects cultured neurons from ischemia-like injury. Exp Neurol. 189:122–30. DOI:
10.1016/j.expneurol.2004.05.029. PMID:
15296842.

27. Freitas AE, Neis VB, Rodrigues ALS. 2016; Agmatine, a potential novel therapeutic strategy for depression. Eur Neuropsychopharmacol. 26:1885–99. DOI:
10.1016/j.euroneuro.2016.10.013. PMID:
27836390.

28. Regunathan S, Feinstein DL, Raasch W, Reis DJ. 1995; Agmatine (decarboxylated arginine) is synthesized and stored in astrocytes. Neuroreport. 6:1897–900. DOI:
10.1097/00001756-199510020-00018. PMID:
8547593.

29. Mancinelli L, Ragonese F, Cataldi S, Ceccarini MR, Iannitti RG, Arcuri C, Fioretti B. 2018; Inhibitory effects of agmatine on monoamine oxidase (MAO) activity: reconciling the discrepancies. EuroBiotech J. 2:121–7. DOI:
10.2478/ebtj-2018-0016.

30. Regunathan S, Piletz JE. 2003; Regulation of inducible nitric oxide synthase and agmatine synthesis in macrophages and astrocytes. Ann N Y Acad Sci. 1009:20–9. DOI:
10.1196/annals.1304.002. PMID:
15028566.

31. Liu Y, Lu GY, Chen WQ, Li YF, Wu N, Li J. 2018; Agmatine inhibits chronic morphine exposure-induced impairment of hippocampal neural progenitor proliferation in adult rats. Eur J Pharmacol. 818:50–6. DOI:
10.1016/j.ejphar.2017.10.018. PMID:
29031903.

32. Kim JH, Kim JY, Jung JY, Lee YW, Lee WT, Huh SK, Lee JE. 2017; Endogenous agmatine induced by ischemic preconditioning regulates ischemic tolerance following cerebral ischemia. Exp Neurobiol. 26:380–9. DOI:
10.5607/en.2017.26.6.380. PMID:
29302205. PMCID:
PMC5746503.

33. Feng Y, Piletz JE, Leblanc MH. 2002; Agmatine suppresses nitric oxide production and attenuates hypoxic-ischemic brain injury in neonatal rats. Pediatr Res. 52:606–11. DOI:
10.1203/00006450-200210000-00023. PMID:
12357058.

34. Moon SU, Kwon KH, Kim JH, Bokara KK, Park KA, Lee WT, Lee JE. 2010; Recombinant hexahistidine arginine decarboxylase (hisADC) induced endogenous agmatine synthesis during stress. Mol Cell Biochem. 345:53–60. DOI:
10.1007/s11010-010-0559-6. PMID:
20730478.

35. Mun CH, Lee WT, Park KA, Lee JE. 2010; Regulation of endothelial nitric oxide synthase by agmatine after transient global cerebral ischemia in rat brain. Anat Cell Biol. 43:230–40. DOI:
10.5115/acb.2010.43.3.230. PMID:
21212863. PMCID:
PMC3015041.

36. Jung HJ, Yang MZ, Kwon KH, Yenari MA, Choi YJ, Lee WT, Park KA, Lee JE. 2010; Endogenous agmatine inhibits cerebral vascular matrix metalloproteinases expression by regulating activating transcription factor 3 and endothelial nitric oxide synthesis. Curr Neurovasc Res. 7:201–12. DOI:
10.2174/156720210792231804. PMID:
20560878.
37. Kim JY, Kim JY, Kim JH, Jung H, Lee WT, Lee JE. 2019; Restorative mechanism of neural progenitor cells overexpressing arginine decarboxylase genes following ischemic injury. Exp Neurobiol. 28:85–103. DOI:
10.5607/en.2019.28.1.85. PMID:
30853827. PMCID:
PMC6401554.

38. Bokara KK, Kwon KH, Nho Y, Lee WT, Park KA, Lee JE. 2011; Retroviral expression of arginine decarboxylase attenuates oxidative burden in mouse cortical neural stem cells. Stem Cells Dev. 20:527–37. DOI:
10.1089/scd.2010.0312. PMID:
20735257.

39. Cameron HA, Woolley CS, McEwen BS, Gould E. 1993; Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 56:337–44. DOI:
10.1016/0306-4522(93)90335-D. PMID:
8247264.

42. Christie BR, Cameron HA. 2006; Neurogenesis in the adult hippocampus. Hippocampus. 16:199–207. DOI:
10.1002/hipo.20151. PMID:
16411231.

44. Goudarzi G, Hamidabadi HG, Bojnordi MN, Hedayatpour A, Niapour A, Zahiri M, Absalan F, Darabi S. 2020; Role of cerebrospinal fluid in differentiation of human dental pulp stem cells into neuron-like cells. Anat Cell Biol. 53:292–300. DOI:
10.5115/acb.19.241. PMID:
32993279. PMCID:
PMC7527124.

45. Haratizadeh S, Nazm Bojnordi M, Darabi S, Karimi N, Naghikhani M, Ghasemi Hamidabadi H, Seifi M. 2017; Condition medium of cerebrospinal fluid and retinoic acid induces the transdifferentiation of human dental pulp stem cells into neuroglia and neural like cells. Anat Cell Biol. 50:107–14. DOI:
10.5115/acb.2017.50.2.107. PMID:
28713614. PMCID:
PMC5509894.

46. Chu MS, Chang CF, Yang CC, Bau YC, Ho LL, Hung SC. 2006; Signalling pathway in the induction of neurite outgrowth in human mesenchymal stem cells. Cell Signal. 18:519–30. DOI:
10.1016/j.cellsig.2005.05.018. PMID:
16098715.

47. Goh EL, Ma D, Ming GL, Song H. 2003; Adult neural stem cells and repair of the adult central nervous system. J Hematother Stem Cell Res. 12:671–9. DOI:
10.1089/15258160360732696. PMID:
14977476.

49. Cho JM, Shin YJ, Park JM, Kim J, Lee MY. 2013; Characterization of nestin expression in astrocytes in the rat hippocampal CA1 region following transient forebrain ischemia. Anat Cell Biol. 46:131–40. DOI:
10.5115/acb.2013.46.2.131. PMID:
23869260. PMCID:
PMC3713277.

50. Anacker C, Hen R. 2017; Adult hippocampal neurogenesis and cognitive flexibility - linking memory and mood. Nat Rev Neurosci. 18:335–46. DOI:
10.1038/nrn.2017.45. PMID:
28469276. PMCID:
PMC6261347.

52. Walz R, Castro RM, Velasco TR, Carlotti CG Jr, Sakamoto AC, Brentani RR, Martins VR. 2002; Cellular prion protein: implications in seizures and epilepsy. Cell Mol Neurobiol. 22:249–57. DOI:
10.1023/A:1020711700048. PMID:
12469868.
54. Deng W, Aimone JB, Gage FH. 2010; New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 11:339–50. DOI:
10.1038/nrn2822. PMID:
20354534. PMCID:
PMC2886712.

55. Drew MR, Hen R. 2007; Adult hippocampal neurogenesis as target for the treatment of depression. CNS Neurol Disord Drug Targets. 6:205–18. DOI:
10.2174/187152707780619353. PMID:
17511617.

56. Nacher J, Alonso-Llosa G, Rosell DR, McEwen BS. 2003; NMDA receptor antagonist treatment increases the production of new neurons in the aged rat hippocampus. Neurobiol Aging. 24:273–84. DOI:
10.1016/S0197-4580(02)00096-9. PMID:
12498961.

58. Seo S, Liu P, Leitch B. 2011; Spatial learning-induced accumulation of agmatine and glutamate at hippocampal CA1 synaptic terminals. Neuroscience. 192:28–36. DOI:
10.1016/j.neuroscience.2011.07.007. PMID:
21777660.

59. Rushaidhi M, Jing Y, Zhang H, Liu P. 2013; Participation of hippocampal agmatine in spatial learning: an
in vivo microdialysis study. Neuropharmacology. 65:200–5. DOI:
10.1016/j.neuropharm.2012.10.007. PMID:
23116777.
60. Song HW, Kumar BK, Kim SH, Jeon YH, Lee YA, Lee WT, Park KA, Lee JE. 2011; Agmatine enhances neurogenesis by increasing ERK1/2 expression, and suppresses astrogenesis by decreasing BMP 2,4 and SMAD 1,5,8 expression in subventricular zone neural stem cells. Life Sci. 89:439–49. DOI:
10.1016/j.lfs.2011.07.003. PMID:
21843531.

61. Berridge MJ, Lipp P, Bootman MD. 2000; The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 1:11–21. DOI:
10.1038/35036035. PMID:
11413485.

62. Humeau J, Bravo-San Pedro JM, Vitale I, Nuñez L, Villalobos C, Kroemer G, Senovilla L. 2018; Calcium signaling and cell cycle: progression or death. Cell Calcium. 70:3–15. DOI:
10.1016/j.ceca.2017.07.006. PMID:
28801101.

63. Green HF, Treacy E, Keohane AK, Sullivan AM, O'Keeffe GW, Nolan YM. 2012; A role for interleukin-1β in determining the lineage fate of embryonic rat hippocampal neural precursor cells. Mol Cell Neurosci. 49:311–21. DOI:
10.1016/j.mcn.2012.01.001. PMID:
22270046.

64. Song J, Kumar BK, Kang S, Park KA, Lee WT, Lee JE. 2013; The effect of agmatine on expression of IL-1β and TLX which promotes neuronal differentiation in lipopolysaccharide-treated neural progenitors. Exp Neurobiol. 22:268–76. DOI:
10.5607/en.2013.22.4.268. PMID:
24465142. PMCID:
PMC3897688.

65. Zunszain PA, Anacker C, Cattaneo A, Choudhury S, Musaelyan K, Myint AM, Thuret S, Price J, Pariante CM. 2012; Interleukin-1β: a new regulator of the kynurenine pathway affecting human hippocampal neurogenesis. Neuropsychopharmacology. 37:939–49. DOI:
10.1038/npp.2011.277. PMID:
22071871. PMCID:
PMC3280640.

66. Mullen RJ, Buck CR, Smith AM. 1992; NeuN, a neuronal specific nuclear protein in vertebrates. Development. 116:201–11. DOI:
10.1242/dev.116.1.201. PMID:
1483388.

67. Cimadamore F, Amador-Arjona A, Chen C, Huang CT, Terskikh AV. 2013; SOX2-LIN28/let-7 pathway regulates proliferation and neurogenesis in neural precursors. Proc Natl Acad Sci U S A. 110:E3017–26. DOI:
10.1073/pnas.1220176110. PMID:
23884650. PMCID:
PMC3740872.

68. Sung SM, Jung DS, Kwon CH, Park JY, Kang SK, Kim YK. 2007; Hypoxia/reoxygenation stimulates proliferation through PKC-dependent activation of ERK and Akt in mouse neural progenitor cells. Neurochem Res. 32:1932–9. DOI:
10.1007/s11064-007-9390-1. PMID:
17562163.

69. Song J, Oh Y, Kim JY, Cho KJ, Lee JE. 2016; Suppression of microRNA let-7a expression by agmatine regulates neural stem cell differentiation. Yonsei Med J. 57:1461–7. DOI:
10.3349/ymj.2016.57.6.1461. PMID:
27593875. PMCID:
PMC5011279.
70. Fu X, Brown KJ, Yap CC, Winckler B, Jaiswal JK, Liu JS. 2013; Doublecortin (Dcx) family proteins regulate filamentous actin structure in developing neurons. J Neurosci. 33:709–21. DOI:
10.1523/JNEUROSCI.4603-12.2013. PMID:
23303949. PMCID:
PMC3711551.

71. Morrissey J, McCracken R, Ishidoya S, Klahr S. 1995; Partial cloning and characterization of an arginine decarboxylase in the kidney. Kidney Int. 47:1458–61. DOI:
10.1038/ki.1995.204. PMID:
7543626.

72. Tocharus C, Puriboriboon Y, Junmanee T, Tocharus J, Ekthuwapranee K, Govitrapong P. 2014; Melatonin enhances adult rat hippocampal progenitor cell proliferation via ERK signaling pathway through melatonin receptor. Neuroscience. 275:314–21. DOI:
10.1016/j.neuroscience.2014.06.026. PMID:
24956284.

74. Kim JH, Kim JY, Mun CH, Suh M, Lee JE. 2017; Agmatine modulates the phenotype of macrophage acute phase after spinal cord injury in rats. Exp Neurobiol. 26:278–86. DOI:
10.5607/en.2017.26.5.278. PMID:
29093636. PMCID:
PMC5661060.

75. Gilad GM, Gilad VH, Finberg JP, Rabey JM. 2005; Neurochemical evidence for agmatine modulation of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) neurotoxicity. Neurochem Res. 30:713–9. DOI:
10.1007/s11064-005-6865-9. PMID:
16187208.

76. Kang S, Kim CH, Jung H, Kim E, Song HT, Lee JE. 2017; Agmatine ameliorates type 2 diabetes induced-Alzheimer's disease-like alterations in high-fat diet-fed mice via reactivation of blunted insulin signalling. Neuropharmacology. 113(Pt A):467–79. DOI:
10.1016/j.neuropharm.2016.10.029. PMID:
27810390.

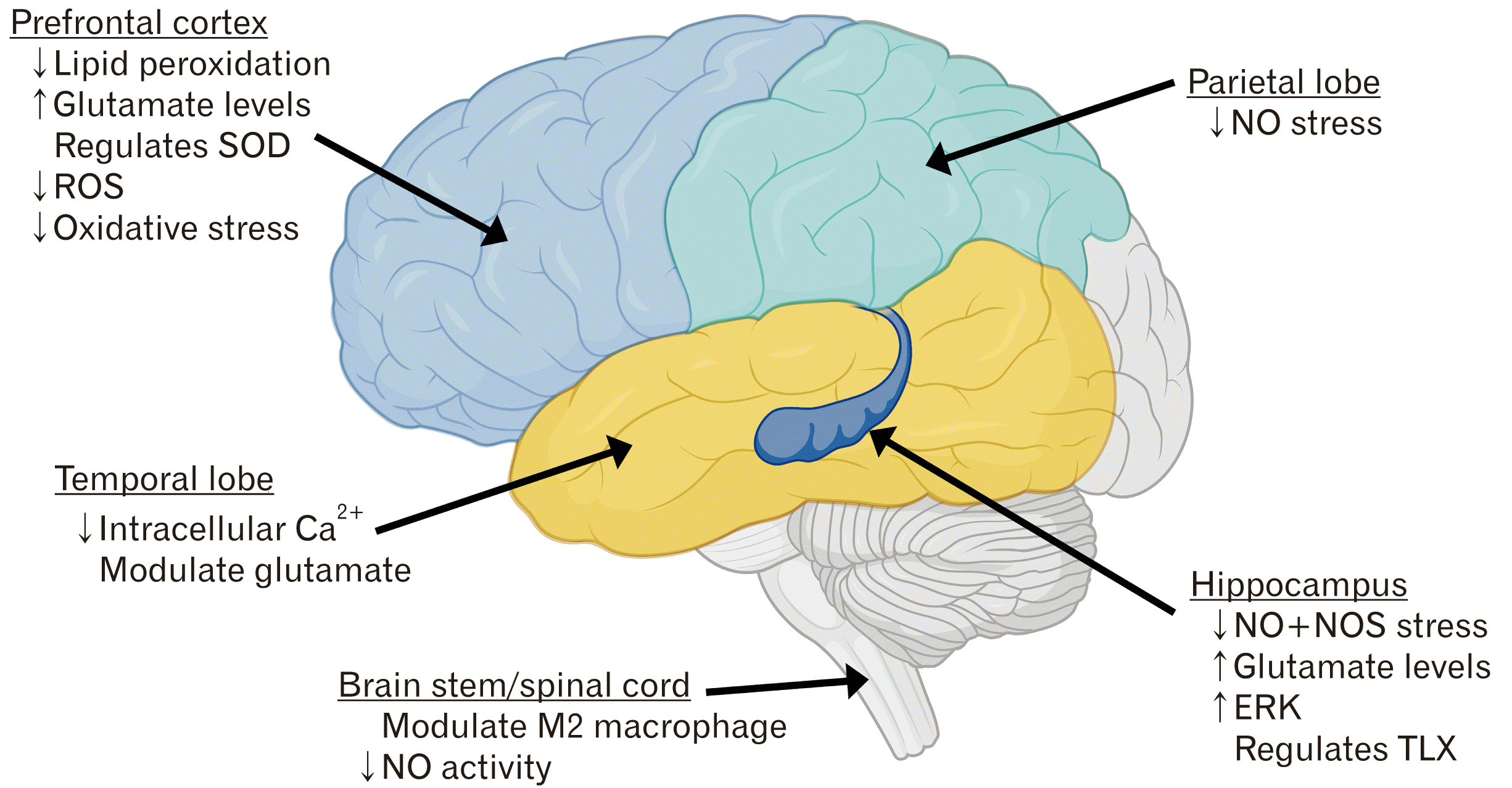
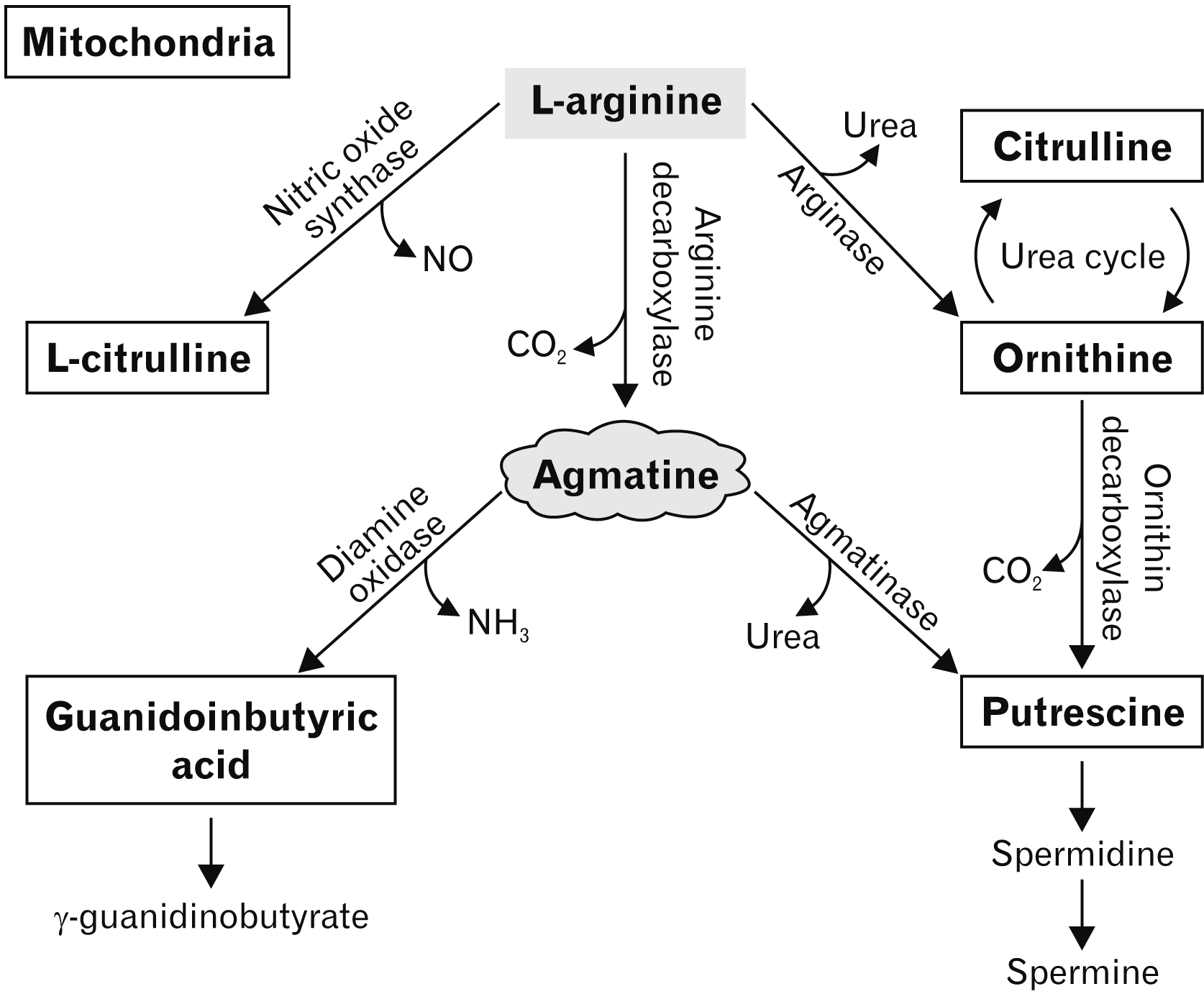




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download