2. Chen QM, Bartholomew JC, Campisi J, Acosta M, Reagan JD, Ames BN. 1998; Molecular analysis of H
2O
2-induced senescent-like growth arrest in normal human fibroblasts: p53 and Rb control G1 arrest but not cell replication. Biochem J. 332(Pt 1):43–50. DOI:
10.1042/bj3320043. PMID:
9576849. PMCID:
PMC1219449.
3. Frippiat C, Dewelle J, Remacle J, Toussaint O. 2002; Signal transduction in H
2O
2-induced senescence-like phenotype in human diploid fibroblasts. Free Radic Biol Med. 33:1334–1346. DOI:
10.1016/S0891-5849(02)01044-4. PMID:
12419465.
4. MacNee W. 2005; Pulmonary and systemic oxidant/antioxidant imbalance in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2:50–60. DOI:
10.1513/pats.200411-056SF. PMID:
16113469.

5. Rahman I, Biswas SK, Kode A. 2006; Oxidant and antioxidant balance in the airways and airway diseases. Eur J Pharmacol. 533:222–239. DOI:
10.1016/j.ejphar.2005.12.087. PMID:
16500642.

6. Han J, Miyamae Y, Shigemori H, Isoda H. 2010; Neuroprotective effect of 3,5-di-O-caffeoylquinic acid on SH-SY5Y cells and senescence-accelerated-prone mice 8 through the up-regulation of phosphoglycerate kinase-1. Neuroscience. 169:1039–1045. DOI:
10.1016/j.neuroscience.2010.05.049. PMID:
20570715.

7. Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, Guarente L, Gu W. 2001; Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 107:137–148. DOI:
10.1016/S0092-8674(01)00524-4. PMID:
11672522.
8. Ota H, Akishita M, Eto M, Iijima K, Kaneki M, Ouchi Y. 2007; SIRT1 modulates premature senescence-like phenotype in human endothelial cells. J Mol Cell Cardiol. 43:571–579. DOI:
10.1016/j.yjmcc.2007.08.008. PMID:
17916362.

10. Ota H, Tokunaga E, Chang K, Hikasa M, Iijima K, Eto M, Kozaki K, Akishita M, Ouchi Y, Kaneki M. 2006; SIRT1 inhibitor, Sirtinol, induces senescence-like growth arrest with attenuated Ras-MAPK signaling in human cancer cells. Oncogene. 25:176–185. DOI:
10.1038/sj.onc.1209049. PMID:
16170353.

11. Ju W, Wang X, Shi H, Chen W, Belinsky SA, Lin Y. 2007; A critical role of luteolin-induced reactive oxygen species in blockage of tumor necrosis factor-activated nuclear factor-kappaB pathway and sensitization of apoptosis in lung cancer cells. Mol Pharmacol. 71:1381–1388. DOI:
10.1124/mol.106.032185. PMID:
17296806.
12. Shi RX, Ong CN, Shen HM. 2004; Luteolin sensitizes tumor necrosis factor-alpha-induced apoptosis in human tumor cells. Oncogene. 23:7712–7721. DOI:
10.1038/sj.onc.1208046. PMID:
15334063.
13. Xagorari A, Papapetropoulos A, Mauromatis A, Economou M, Fotsis T, Roussos C. 2001; Luteolin inhibits an endotoxin-stimulated phosphorylation cascade and proinflammatory cytokine production in macrophages. J Pharmacol Exp Ther. 296:181–187. PMID:
11123379.
15. Kang KP, Park SK, Kim DH, Sung MJ, Jung YJ, Lee AS, Lee JE, Ramkumar KM, Lee S, Park MH, Roh SG, Kim W. 2011; Luteolin ameliorates cisplatin-induced acute kidney injury in mice by regulation of p53-dependent renal tubular apoptosis. Nephrol Dial Transplant. 26:814–822. DOI:
10.1093/ndt/gfq528. PMID:
20817674.

16. Jang S, Dilger RN, Johnson RW. 2010; Luteolin inhibits microglia and alters hippocampal-dependent spatial working memory in aged mice. J Nutr. 140:1892–1898. DOI:
10.3945/jn.110.123273. PMID:
20685893. PMCID:
PMC2937579.

17. Xu B, Li XX, He GR, Hu JJ, Mu X, Tian S, Du GH. 2010; Luteolin promotes long-term potentiation and improves cognitive functions in chronic cerebral hypoperfused rats. Eur J Pharmacol. 627:99–105. DOI:
10.1016/j.ejphar.2009.10.038. PMID:
19857483.

18. Kalinec GM, Webster P, Lim DJ, Kalinec F. 2003; A cochlear cell line as an
in vitro system for drug ototoxicity screening. Audiol Neurootol. 8:177–189. DOI:
10.1159/000071059. PMID:
12811000.
19. Yu HB, Li DY, Zhang HF, Xue HZ, Pan CE, Zhao SH, Wang L. 2010; Resveratrol inhibits invasion and metastasis of hepatocellular carcinoma cells. J Anim Vet Adv. 9:3117–3124. DOI:
10.3923/javaa.2010.3117.3124.

20. Lee DS, Li B, Keo S, Kim KS, Jeong GS, Oh H, Kim YC. 2013; Inhibitory effect of 9-hydroxy-6,7-dimethoxydalbergiquinol from Dalbergia odorifera on the NF-κB-related neuroinflammatory response in lipopolysaccharide-stimulated mouse BV2 microglial cells is mediated by heme oxygenase-1. Int Immunopharmacol. 17:828–835. DOI:
10.1016/j.intimp.2013.08.024. PMID:
24055019.

21. Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, Peacocke M, Campisi J. 1995; A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 92:9363–9367. DOI:
10.1073/pnas.92.20.9363. PMID:
7568133. PMCID:
PMC40985.

22. Cheng XS, Li MS, Du J, Jiang QY, Wang L, Yan SY, Yu DM, Deng JB. 2011; Neuronal apoptosis in the developing cerebellum. Anat Histol Embryol. 40:21–27. DOI:
10.1111/j.1439-0264.2010.01033.x. PMID:
21231956.

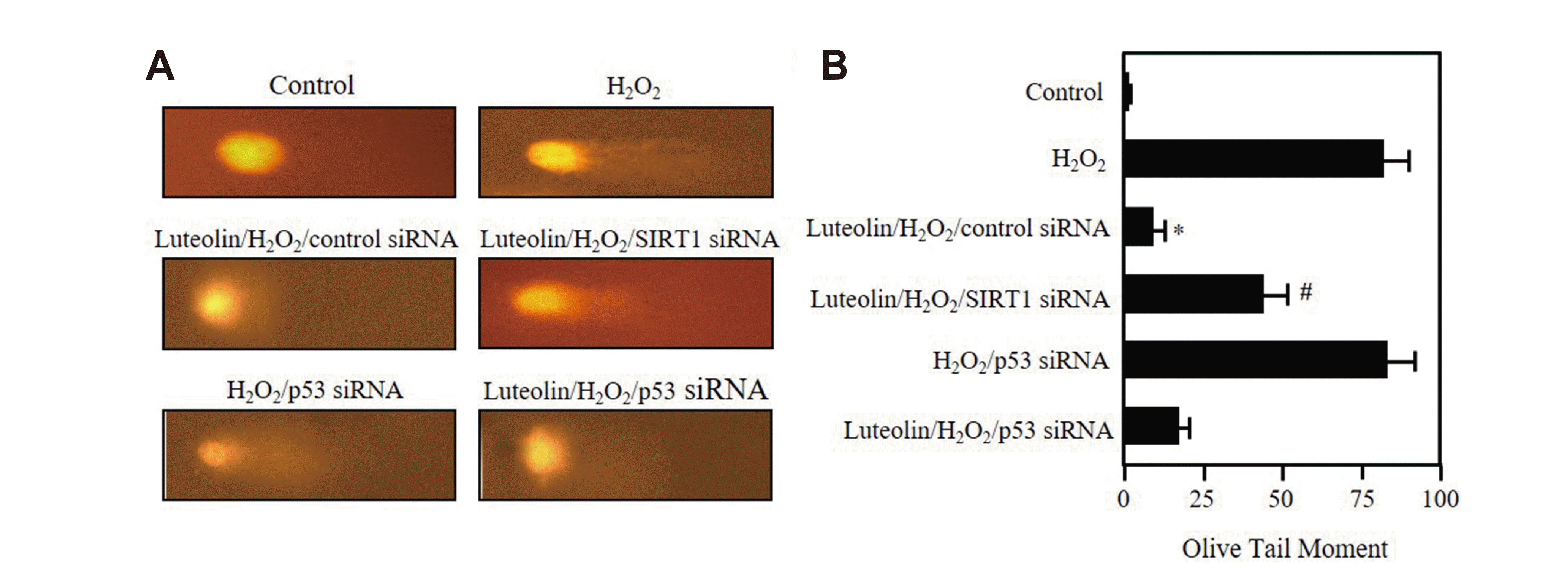
23. Olive PL, Banáth JP. 2006; The comet assay: a method to measure DNA damage in individual cells. Nat Protoc. 1:23–29. DOI:
10.1038/nprot.2006.5. PMID:
17406208.

24. Muller M. 2009; Cellular senescence: molecular mechanisms,
in vivo significance, and redox considerations. Antioxid Redox Signal. 11:59–98. DOI:
10.1089/ars.2008.2104. PMID:
18976161.
25. Andrade LN, Nathanson JL, Yeo GW, Menck CF, Muotri AR. 2012; Evidence for premature aging due to oxidative stress in iPSCs from Cockayne syndrome. Hum Mol Genet. 21:3825–3834. DOI:
10.1093/hmg/dds211. PMID:
22661500. PMCID:
PMC3412382.

26. Tilstra JS, Robinson AR, Wang J, Gregg SQ, Clauson CL, Reay DP, Nasto LA, St Croix CM, Usas A, Vo N, Huard J, Clemens PR, Stolz DB, Guttridge DC, Watkins SC, Garinis GA, Wang Y, Niedernhofer LJ, Robbins PD. 2012; NF-κB inhibition delays DNA damage-induced senescence and aging in mice. J Clin Invest. 122:2601–2612. DOI:
10.1172/JCI45785. PMID:
22706308. PMCID:
PMC3386805.

27. Wölfle U, Esser PR, Simon-Haarhaus B, Martin SF, Lademann J, Schempp CM. 2011; UVB-induced DNA damage, generation of reactive oxygen species, and inflammation are effectively attenuated by the flavonoid luteolin
in vitro and
in vivo. Free Radic Biol Med. 50:1081–1093. DOI:
10.1016/j.freeradbiomed.2011.01.027. PMID:
21281711.
28. Rodier F, Campisi J, Bhaumik D. 2007; Two faces of p53: aging and tumor suppression. Nucleic Acids Res. 35:7475–7484. DOI:
10.1093/nar/gkm744. PMID:
17942417. PMCID:
PMC2190721.

29. Mao GX, Wang Y, Qiu Q, Deng HB, Yuan LG, Li RG, Song DQ, Li YY, Li DD, Wang Z. 2010; Salidroside protects human fibroblast cells from premature senescence induced by H
2O
2 partly through modulating oxidative status. Mech Ageing Dev. 131:723–731. DOI:
10.1016/j.mad.2010.10.003. PMID:
21035481.
30. Duan J, Duan J, Zhang Z, Tong T. 2005; Irreversible cellular senescence induced by prolonged exposure to H
2O
2 involves DNA-damage-and-repair genes and telomere shortening. Int J Biochem Cell Biol. 37:1407–1420. DOI:
10.1016/j.biocel.2005.01.010. PMID:
15833273.
31. Rajendran R, Garva R, Krstic-Demonacos M, Demonacos C. 2011; Sirtuins: molecular traffic lights in the crossroad of oxidative stress, chromatin remodeling, and transcription. J Biomed Biotechnol. 2011:368276. DOI:
10.1155/2011/368276. PMID:
21912480. PMCID:
PMC3168296.

32. Chua KF, Mostoslavsky R, Lombard DB, Pang WW, Saito S, Franco S, Kaushal D, Cheng HL, Fischer MR, Stokes N, Murphy MM, Appella E, Alt FW. 2005; Mammalian SIRT1 limits replicative life span in response to chronic genotoxic stress. Cell Metab. 2:67–76. DOI:
10.1016/j.cmet.2005.06.007. PMID:
16054100.

33. Lim CS, Potts M, Helm RF. 2006; Nicotinamide extends the replicative life span of primary human cells. Mech Ageing Dev. 127:511–514. DOI:
10.1016/j.mad.2006.02.001. PMID:
16545428.

34. Chung S, Yao H, Caito S, Hwang JW, Arunachalam G, Rahman I. 2010; Regulation of SIRT1 in cellular functions: role of polyphenols. Arch Biochem Biophys. 501:79–90. DOI:
10.1016/j.abb.2010.05.003. PMID:
20450879. PMCID:
PMC2930135.

35. Kaeberlein M, McDonagh T, Heltweg B, Hixon J, Westman EA, Caldwell SD, Napper A, Curtis R, DiStefano PS, Fields S, Bedalov A, Kennedy BK. 2005; Substrate-specific activation of sirtuins by resveratrol. J Biol Chem. 280:17038–17045. DOI:
10.1074/jbc.M500655200. PMID:
15684413.

36. de Boer VC, de Goffau MC, Arts IC, Hollman PC, Keijer J. 2006; SIRT1 stimulation by polyphenols is affected by their stability and metabolism. Mech Ageing Dev. 127:618–627. DOI:
10.1016/j.mad.2006.02.007. PMID:
16603228.

37. Davis JM, Murphy EA, Carmichael MD, Davis B. 2009; Quercetin increases brain and muscle mitochondrial biogenesis and exercise tolerance. Am J Physiol Regul Integr Comp Physiol. 296:R1071–R1077. DOI:
10.1152/ajpregu.90925.2008. PMID:
19211721.






 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download