Abstract
Doxorubicin (Dox) is widely used to the treatment of cancer, however, it could cause damage to gastric mucosa. To investigate the protective effects and related mechanisms of coenzyme Q10 (CoQ10) and vitamin C (VC) on Dox-induced gastric mucosal injury, we presented the survey of the 4 groups of the rats with different conditions. The results showed Dox treatment significantly induced GES-1 apoptosis, but preconditioning in GES-1 cells with VC or CoQ10 significantly inhibited the Dox-induced decrease and other harm effects, including the expression and of IκKβ, IκBα, NF-κB/p65 and tumor necrosis factor (TNF-α) in GES-1 cells. Moreover, high-throughput sequencing results showed Dox treatment increased the number of harmful gut microbes, and CoQ10 and VC treatment inhibited this effect. CoQ10 and VC treatment inhibits Dox-induced gastric mucosal injury by inhibiting the activation of the IkKB/IκBα/NF-κB/p65/TNF-α pathway, promoting anti-inflammatory effects of gastric tissue and regulating the composition of the intestinal flora.
Cancer is a common malignant disease, which is a serious threat to human health. According to statistics, 13.1 million people will die of cancer by 2030 [1]. Doxorubicin (Dox), also known as adriamycin, has the advantages of a wide antitumor spectrum, strong activity and low price and has been used to treat various types of cancer [2,3]. However, its clinical application is limited due to its side effects, such as nausea, vomiting, liver function injury, and myocardial injury. The mechanism of action of doxorubicin is complex; one of the mechanisms of action is the generation of free radicals that induce DNA damage and cell membrane damage [4]. Coenzyme Q10 (CoQ10) is a fat-soluble quinone compound that is present in a wide range of biological cells and has an antioxidant effect. Vitamin C (VC) is a water-soluble antioxidant that relieves lipid peroxidation and removes oxidative free radicals from water-soluble components. Different types of cancer tissues exhibit different basal redox states, and redox-based anticancer therapy research has emerged. For example, triple-negative breast cancer cell lines are sensitive to the combination of auranofin and VC [5]. Regulation of the oxidative balance with CoQ10 can make human glioblastoma cells sensitive to radiation and temozolomide [6]. Chicken embryonic amniotic fluid and coenzyme Q10 can compete with vitamin C and other compounds to increase the antioxidant level in ovarian tissue [7]. 16S rDNA is the characteristic ribosomal coding sequence of prokaryotes that is often used for the classification of bacteria and archaea and the study of evolutionary relationships. Microbial diversity sequencing is a research method that uses high-throughput sequencing technology to detect the characteristic 16S, 18S, ITS and other microbial DNA sequences amplified by PCR. In this paper, the protective effect of coenzyme Q10 and vitamin C on doxorubicin-induced gastric mucosal injury and related mechanisms was explored through cell experiments and animal experiments, providing theoretical support for cancer treatment.
The animal experiment was approved by the ethics committee of Tianjin Medical University (approval Number: TMUaMEC2019012). All procedures were performed in accordance with national (D.L.n.26, March 4th, 2014) and international laws and policies (directive 2010/63/EU).
Wistar rats were purchased from Hunan Slex Laboratory Animal Co., Ltd, China. GES-1 cells were purchased from Shanghai Fuheng Biotechnology Co., Ltd, China. PCR primers and RNA extraction and reverse transcription kits were purchased from Invitrogen. Real-time PCR reagents were purchased from Promega, Madison, WI, USA; Hematoxylin-eosin, CoQ10 and VC were purchased from Sigma, Shanghai, China; Dox was purchased from Pharmacia, Jinan, China. Tumor necrosis factor-α (TNF-α) detection, interleukin-6 (IL-6) detection, reduced glutathione (GSH) assay and micro malondialdehyde (MDA) assay kits were from Nanjing Jiancheng Biological Co., Ltd, China. The interferon-gamma (INF-γ) detection kit was purchased from R&D, TRIzol, DAPI, MTT powder and trypsin were purchased from Beijing Soleil Technology Co., Ltd, China. The BCA protein quantification kit was purchased from Shanghai Biyuntian Biotechnology Research Institute, Chian; DMEM medium and FBS were purchased from Gibco, Grand Island, NY, USA. The antibodies used in the experiments were purchased from Santa Cruz Biotechnology, Inc, Santa Cruz, CA, USA.
Cell culture and treatment dosing: A cryotube containing the GES-1 cells was removed from the liquid nitrogen, quickly placed in a 37°C water bath and gently shaken to rapidly melt the contents. Then, in an aseptic workstation, the frozen solution was transferred to a 5 ml sterile EP tube, centrifuged at 1,000 rpm for 5 min, the supernatant discarded, the pellet resuspended in 2 ml of fresh DMEM and inoculated into a 10 cm Petri dish, which was then cultured in a 5% CO2 incubator at 37°C.
Before dosing treatment, Dox, VC, and CoQ 10 were formulated into stock solutions with concentrations of 16 μM, 25 μg/ml, and 5 μM in FBS-free fresh medium. For cell treatments, the old medium was discarded, and the appropriate amount of fresh medium added. According to the required concentration of each treatment, an appropriate amount of VC or CoQ 10 was added for 24 h, and the blank and the Dox treatment groups were treated with an equal volume of FBS-free medium. After 24 h, the cells were treated with Dox for 24 h, and the blank group cells were supplemented with an equal volume of FBS-free medium.
Total RNA extraction from cells: After 48 h of treatment, the medium from cells was discarded in an aseptic workstation, the cells were washed once with PBS, 1 ml of TRIzol added per well, and cells were lysed on ice for 15 min. After lysis, the cells were suspended by a pipette, then, phenol, chloroform, ethanol and other organic solvents were used to extract the total RNA. The RNA extracted from cells was stored at –20°C saved for later use.
MTT experiment: GES-1 cells in the logarithmic growth phase were digested, counted, and seeded in 96-well plates at 3,000 cells/well, grouped, labeled, and placed in a 37°C, 5% CO2 incubator for 24 h. After 48 h of drug treatment, 20 μl of MTT solution (5 mg/ml) was added per well, and the plate was incubated for 4 h in the incubator. Subsequently, the old medium in the well was discarded, 100 μl of DMSO was added per well, the plate was shaken on a shaker for 10 min, and then the absorbance per well was measured at 490 nm.
TUNEL detection of apoptosis: Coverslips (1 cm × 1 cm) were placed in each well of a 24-well plate. Cells in the logarithmic growth phase were trypsin digested, counted, and seeded in 24-well plates at 2 × 105 cells/well, grouped, labeled, and placed in a 37°C, 5% CO2 incubator for 24 h. Each group of cells was treated with the appropriate drug for 48 h. According to the instructions of the TUNEL kit, the apoptosis of each group was observed by using an inverted fluorescence microscope.
Real-time PCR: According to the instructions of the reverse transcription kit and SYBR Green qPCR kit, relative gene expression was detected by using a Veriti 96-well thermal cycler PCR instrument. The primers used were IκKβ forward primer 5'-CCTGCCACTCAGTGTATTT-3', IκKβ reverse primer 5'-GATTCCTCTTGGGCTCTT-3'; IκBα forward primer 5'-CTGAAGGCTACCAACTACAATG-3', IκBα reverse primer 5'-GCACCCAAGGACACCAAA-3'; P65 forward primer 5'-GGGGACTACGACCTGAATG-3', P65 reverse primer 5'-GGGCACGATTGTCAAAGAT-3'; TNF-α forward primer 5'-CGAGTGACAAGCCTGTAGCC-3', TNF-α reverse primer 5'-TGAAGAGGACCTGGGAGTAGAT-3'; and GAPDH forward primer 5'-ACACCCACTCCTCCACCTTT-3' and GAPDH reverse primer 5'-TTACTCCTTGGAGGCCATGT-3'.
Nuclear protein extraction: Wash with PBS, scrape off the cells with a cell scraper, collect the cells by centrifugation, suck up the supernatant as much as possible, and leave the cell precipitation for later use. Every 20 μl cells were precipitated and 200 μl were added with PMSF cytoplasmic protein extraction reagent A. At the highest speed Vortex 5 sec, the cell precipitate was completely suspended and dispersed, and the ice bath lasted for 10–15 min. Add cytoplasmic protein extraction reagent B 10 μl. Maximum speed vigorous Vortex 5 sec, 1 min ice bath. The highest speed vigorous Vortex was centrifuged for 5 min at 4°C 12,000–16,000 g. The supernatant is immediately absorbed into a pre-cooled plastic tube, which is the cytoplasmic protein extracted. For the precipitation, the residual supernatant was completely absorbed and 50 μl of nuclear protein extraction reagent PMSF was added. The maximum speed of Vortex is 15–30 sec. The precipitate is completely suspended and dispersed. Then return to the ice bath and speed Vortex for 15–30 sec every 1–2 min for a total of 30 min. Centrifuge for 10 min at 4°C 12,000–16,000 g. The supernatant is immediately absorbed into a pre-cooled plastic tube, which is the extracted nuclear protein.
Western blotting: GES-1 cells in the logarithmic growth phase were digested, counted, inoculated into 6-well plates at 1 × 106 cells/well, grouped and labeled. Dosing was performed when the cell confluence reached approximately 85%. Fresh medium was replaced, and then each group was treated with the respective drug for 48 h. The old medium was discarded, 1× protein lysis buffer added, and the cells lysed on the ice for 30 min. After lysis, the cells were scraped off with a scraper, collected in a clean and sterile centrifuge tube, and boiled in boiling water for 10 min. The protein content of the cell lysates was quantified by a BCA protein quantification kit. The samples were loaded for SDS-PAGE at 10 µg per well. The proteins were separated by polyacrylamide gel electrophoresis, transferred to a membrane, blocked with skim milk powder, incubated with antibodies and washed. Finally, the membrane was placed in a Western Lightning Chemiluminescence Reagent developer for 30 sec. The membrane was placed in an exposure chamber, and the photosensitive film was exposed in a dark room and then developed and fixed.
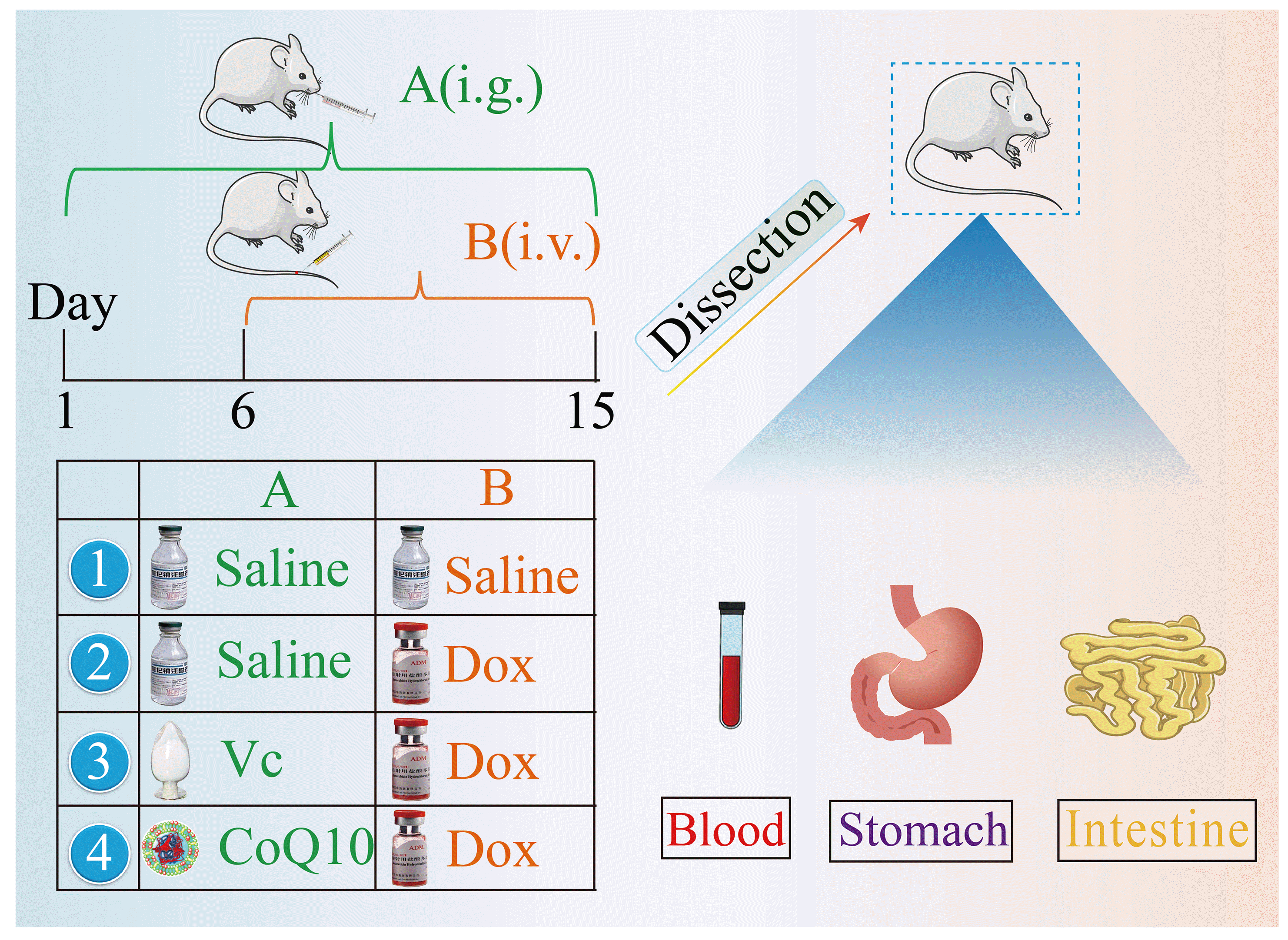
Rat serum and gastric tissue collection: Thirty-two Wistar rats aged 6–8 weeks were purchased; half were male and half female, weighing 185–220 g. After one week of adaptation, 32 rats were randomly divided into 4 groups: blank control group, Dox group, VC + Dox group and CoQ10 + Dox group, with 8 rats in each group. Dox was injected by the tail vein; VC and CoQ10 were administered by gavage. Five days before the injection, the third group and the fourth group were intragastrically treated for 5 days. The first and second groups were intragastrically administered the same volume of normal saline. Dox tail vein injection was given to the second, third and fourth groups from the sixth day. The first group was given the same volume of normal saline. The experiment lasted for 10 days. The schematic diagram of experiments is shown in Fig. 1.
At the end of the experiment, the rats were fasted for 12 h, and the eyeballs were taken by the eyeball method. After blood collection, the rats were sacrificed by the lethal cervical vertebra method. The blood was placed at room temperature for 10 min, centrifuged at 3,000 rpm for 10 min, and the supernatant was aspirated and kept for serum sample testing; the serum was stored at 4°C for use. The rats were sacrificed by the cervical dislocation method, and the contents of the jejunum to the ileum of each rat were aseptically collected in an aseptic workstation, placed in a sterile, ice-box precooled centrifuge tube, and stored at –80°C for use. Gastric tissues of rats were extracted, washed with PBS, and then fixed in liquid nitrogen or 4% paraformaldehyde solution for preservation.
Determination of glutathione (GSH) and MDA contents in gastric tissue: According to the weight (g): volume (ml) = 1:9, the gastric tissues to be tested were accurately weighed. Then, neutral phosphate buffer (0.1 M) was added to tissue homogenate, and the samples were centrifuged at 3,500 rpm for 10 min; the supernatant was taken as the sample to be tested. GSH and MDA content in the labeled and grouped gastric tissues was detected by a GSH assay kit and an MDA assay kit in strict accordance with the manufacturer’s instructions.
Tissue total RNA extraction and real-time PCR: The tissue was removed from the liquid nitrogen tank, and the preserved tissue was placed on an aseptic workstation, ground to powder in liquid nitrogen. and then treated with organic solvents such as phenol, chloroform and ethanol to extract the total RNA of the tissue. The real-time PCR method was as described in “Real-time PCR”. The primers used were as follows: GAPDH forward primer 5'-ATTCAACGGCACAGTCAAGG-3', reverse primer 5'-GCAGAAGGGGCGGAGATGA-3'; TNF-α forward primer 5'-ATTGGCAAATGGGAAAATGA-3', reverse primer 5'-TTATGACCTCCTTTTG GTCTGA-3'; IL-6 forward primer 5'-GACTGATGTTGTTGAGAGCCACTG-3', reverse primer 5'-TAGCCACGCCTTCTGTGACTCTAACT-3'; and INF-γ forward primer 5'-ACTTCTTTGGCTTAATTCTC-3', and reverse primer 5'-ATTGCTTTGCGTTGGA-3'.
ELISA and Western blotting for the detection of TNF-α, IL-6 and INF-γ in serum samples: The serum to be tested was centrifuged at 3,000 rpm for 10 min, and the supernatant was taken for use. Detection kits for TNF-α, IL-6 and INF-γ were used to detect the levels of TNF-α, IL-6 and INF-γ in labeled and grouped rat serum, in strict accordance with the manufacturer’s instructions.
Extraction of the intestinal microbiome, 16S rDNA high-throughput sequencing and intestinal microbial analysis were performed by Guangzhou Kiddio Biotechnology Co., Ltd., Guangzhou, China.
Experimental data were processed using GraphPad Prism 5 software, and the data were expressed as x ± S. Statistical differences between groups were compared using ANOVA: tukey’s test. And the concentration of index differences was compared using t-test.
Different tags are clustered into OTUs using the sequence similarity relationship between valid tags. According to its abundance and sequence information, it is possible to carry out various core analyses such as species annotation, community diversity, and group differences. The Unifrac-based β diversity analysis was used to compare the differences between all samples. The LEfSe analysis was used to determine the difference in microbial groups between Dox and VC + DOX, coenzyme Q10 + Dox.
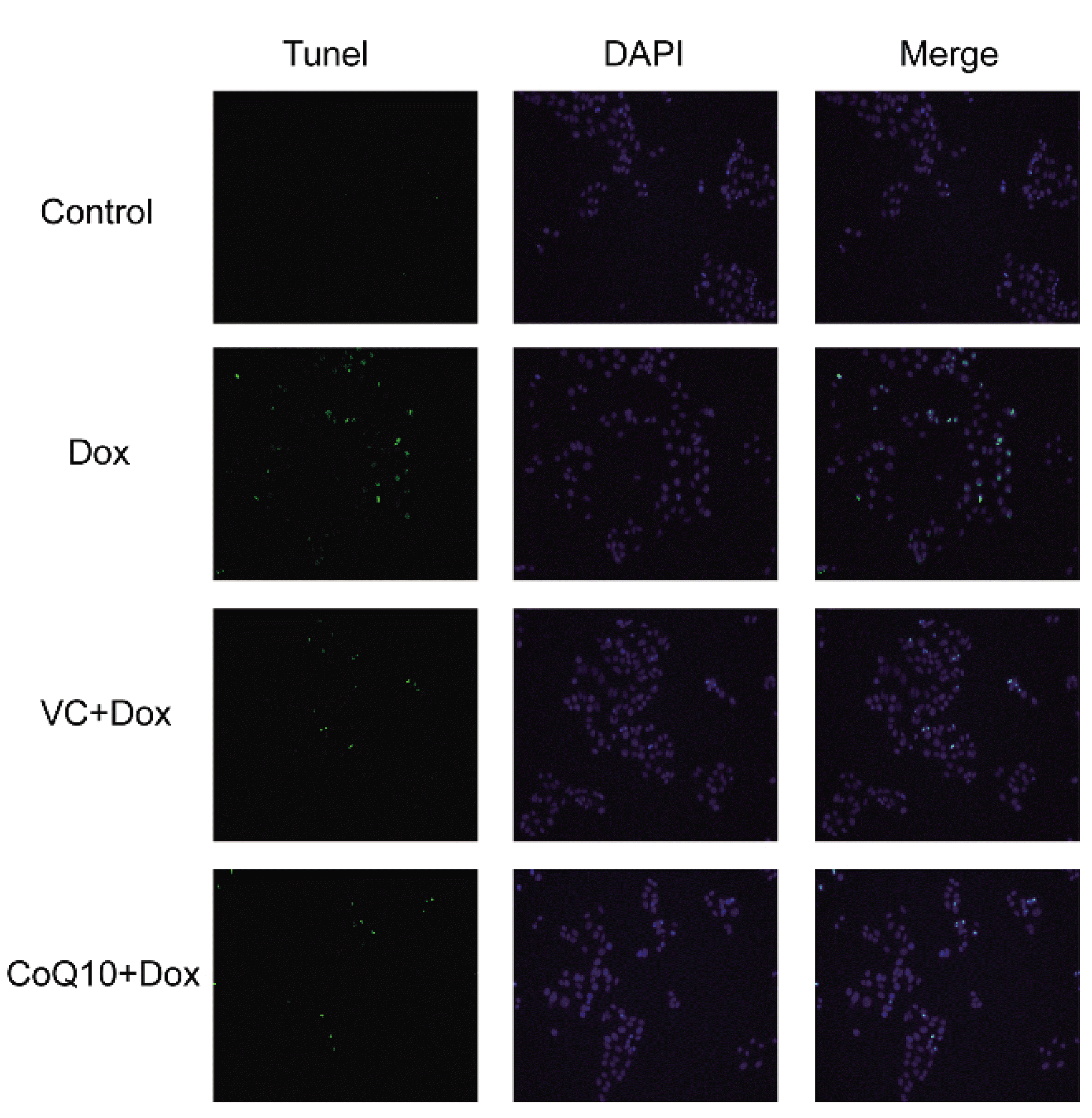
The results of the TUNEL experiment (Fig. 2) showed that Dox stimulation significantly induced apoptosis compared with no stimulation. Apoptosis was significantly decreased in the VC + Dox group and the CoQ10 + Dox group. This demonstrates that VC and CoQ10 treatment inhibited Dox-induced apoptosis of GES-1 cells.
GES-1 cells were stimulated with different concentrations of Dox. The MTT results (Supplementary Fig. 1A) showed that the metabolic activity of GES-1 cells gradually decreased with increasing Dox concentration, and GES-1 cells had the lowest proliferation capacity after Dox treatment at 16 μM. GES-1 cells were stimulated for 24 h with different concentrations of VC and CoQ10 and then treated with 16 μM Dox for 24 h. The MTT results (Supplementary Fig. 1B, C) showed that the proliferation capacity of GES-1 cells gradually increased with increasing VC and CoQ10 concentrations; the optimal concentration of CoQ10 and VC was 5 μM and 25 μg/ml, respectively. Therefore, VC and CoQ10 treatment inhibited the Dox-induced decrease in GES-1 cell proliferation capacity.
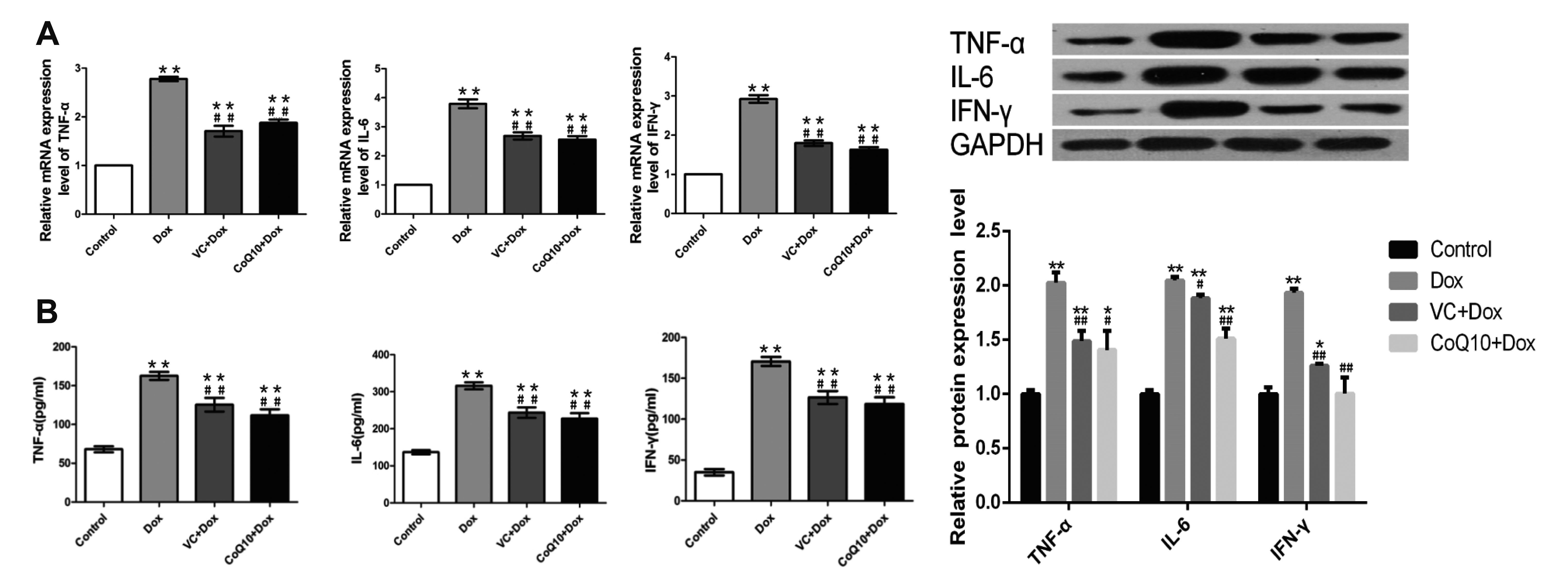
At the mRNA level, the RT-PCR results (Fig. 3, Supplementary Fig. 2) showed that Dox stimulation significantly increased the expression of IκKβ, IκBα, NF-κB/p65, and TNF-α in GES-1 cells. VC and CoQ10 treatment significantly inhibited the increase of IκKβ, IκBα, NF-κB/p65, and TNF-α expression in Dox-induced cells. At the protein level (Fig. 3), Dox stimulation significantly induced an increase in IκKβ, p-IκBα, nuclear NF-κB/p65 and TNF-α expression in GES-1 cells and induced a decrease in cytoplasmic NF-κB/p65 expression. VC and CoQ10 treatment significantly inhibited this effect of Dox.
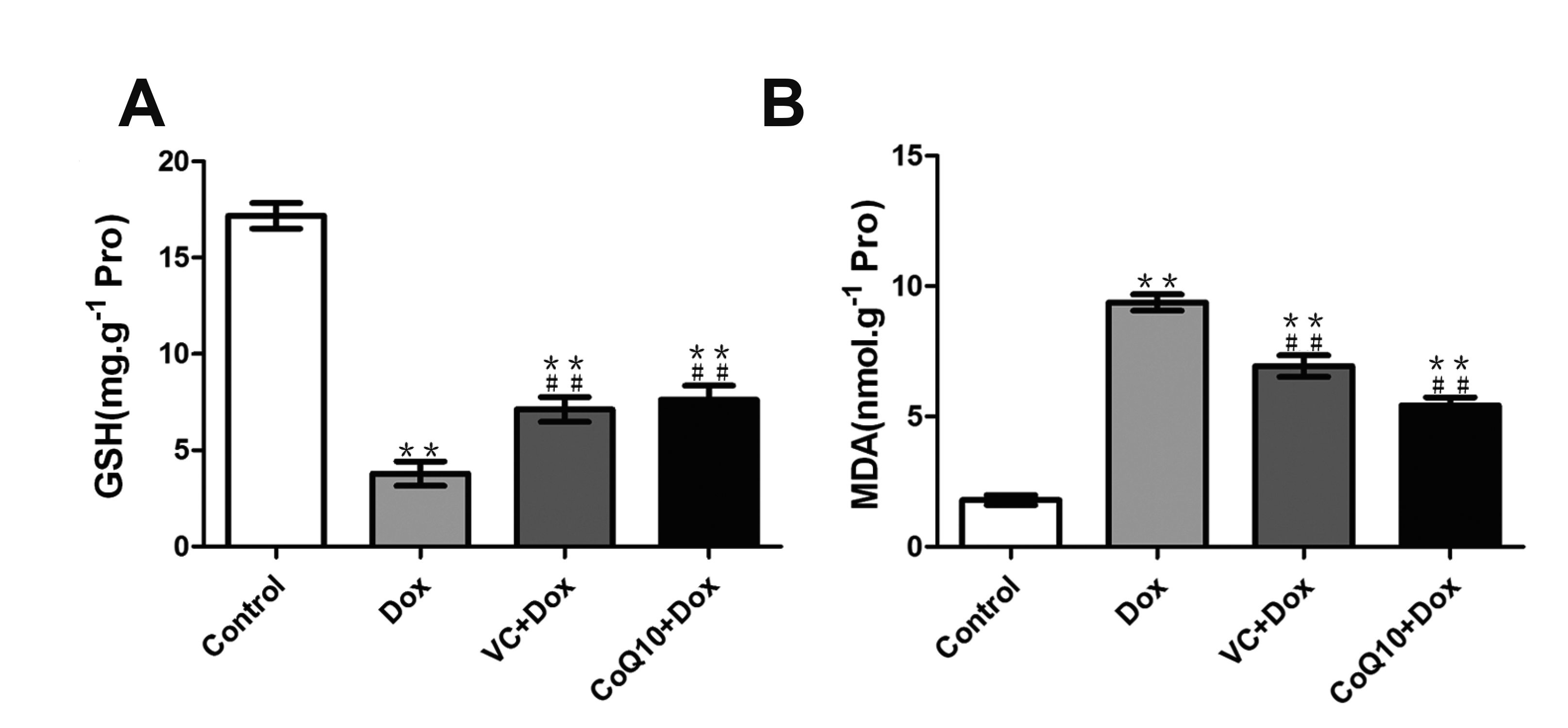
GSH, chemically known as N-(n-l-gamma-l-cysteine) glycine, is an important antioxidant in animal and plant cells. The GSH content in gastric tissue was detected by ELISA. The results (Fig. 4A) showed that the GSH level in gastric tissue of the Dox group was significantly decreased, and CoQ10 and VC treatment significantly inhibited the Dox-induced GSH reduction. Therefore, CoQ10 and VC treatment can upregulate the decrease of GSH in Dox-induced gastric tissues and reduce oxidative damage to gastric tissues.
The mRNA levels of TNF-α, IL-6 and INF-γ mRNA in gastric tissue of each group were detected. The results (Fig. 5) showed that the expression of TNF-α, IL-6 and INF-γ mRNA in the gastric tissue of the Dox-treated group was significantly higher than that in the control group. Compared with the Dox group, the expression of TNF-α, IL-6 and INF-γ mRNA in the VC + Dox and CoQ10 + Dox groups was significantly decreased. The protein levels of TNF-α, IL-6 and INF-γ in the serum of each group were also got the same result. Therefore, CoQ10 and VC significantly reduced the Dox-induced upregulation of TNF-α, IL-6 and INF-γ mRNA.
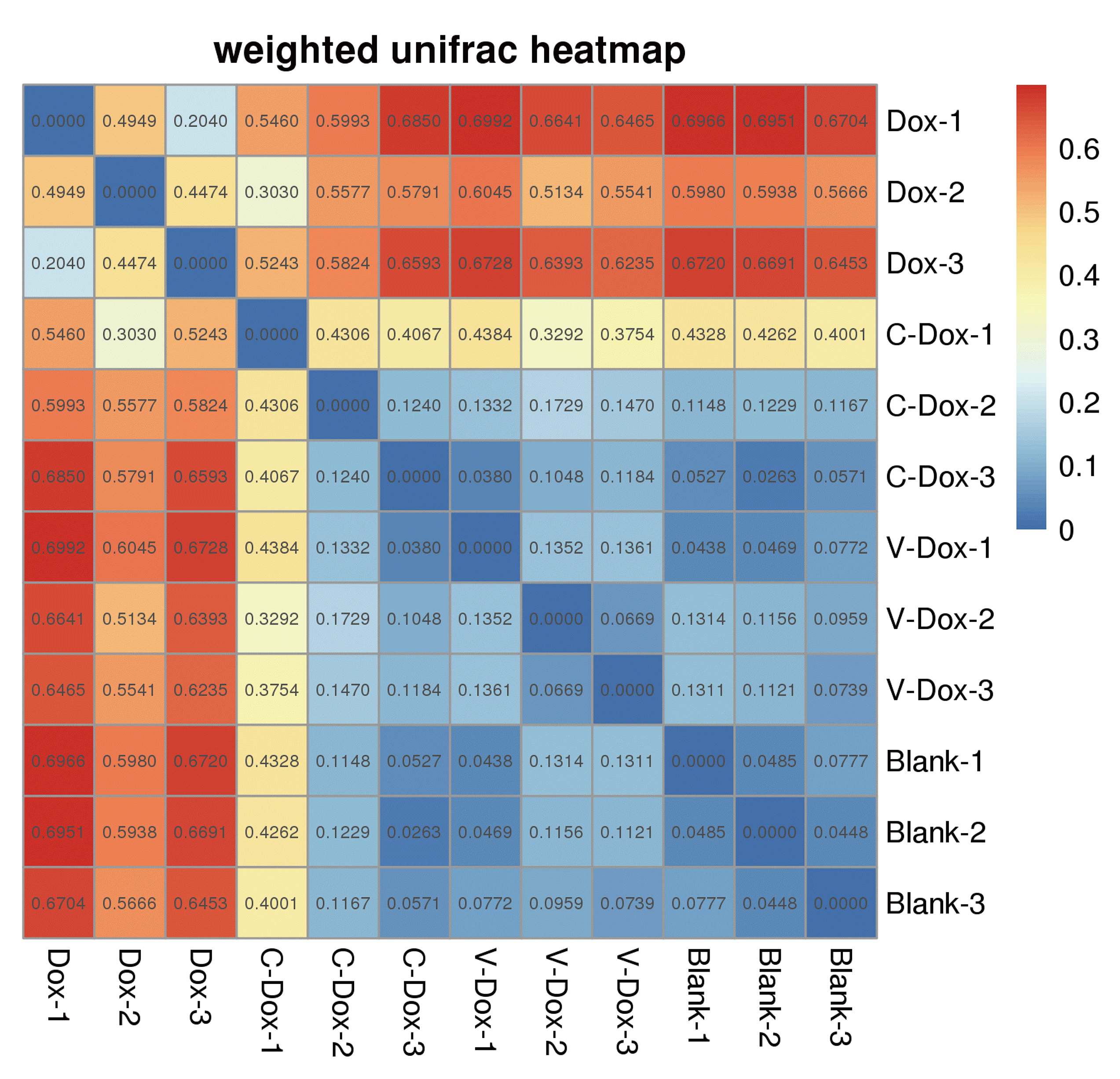
By collecting the intestinal contents of rats and using 16S rDNA sequencing technology to detect microbial composition and diversity. In the study of microbial β diversity diversity, the evolutionary relationship between OTU representative sequences is fully considered, so as to be more in line with the actual biological significance of microbial community changes. It can be seen from the Weighted Unifrac heatmap in Fig. 6 that the Blank group is more closely related to the species and the abundance of species in the CoQ10 + DOX group and the Vc + DOX group.
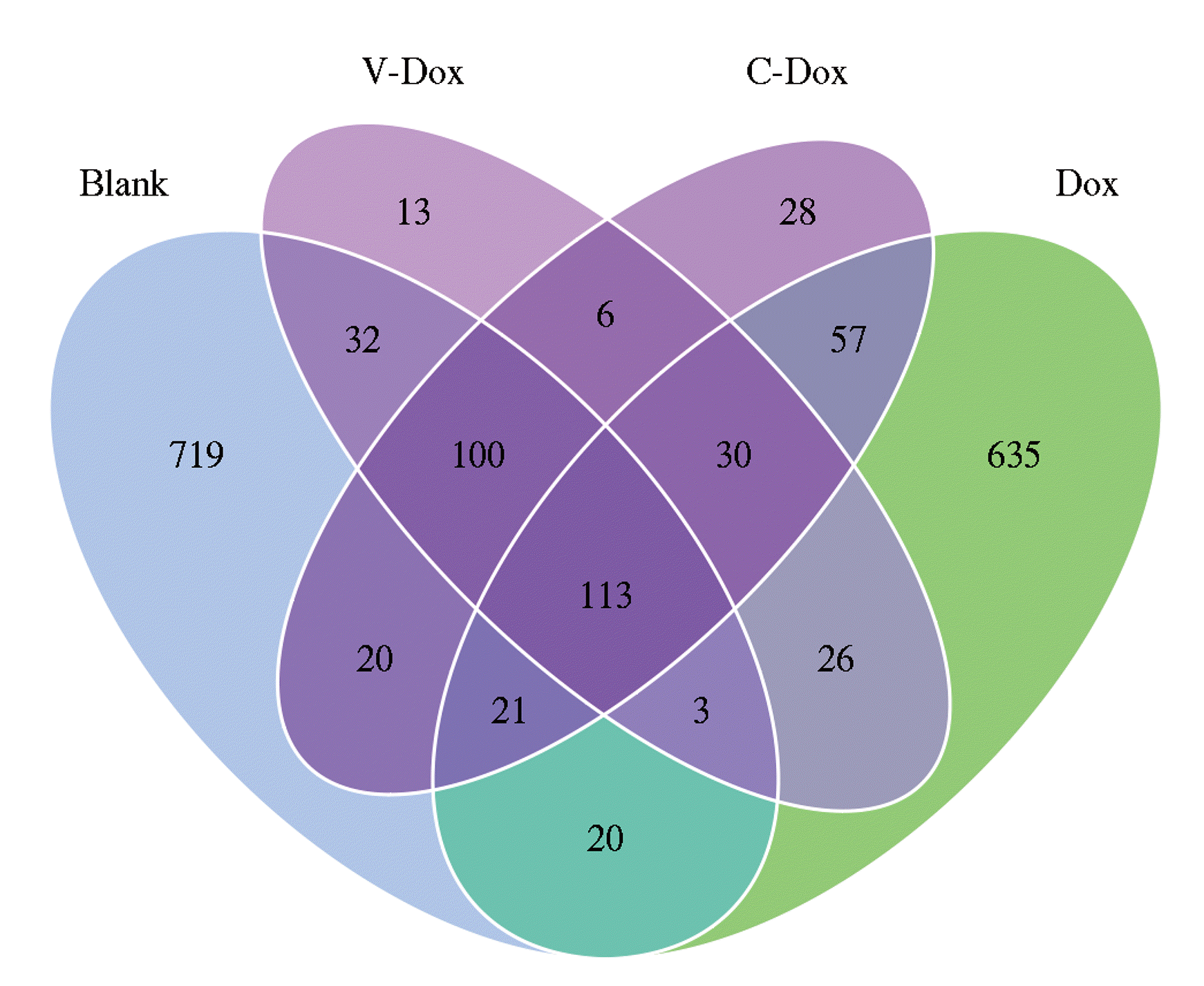
For OTU picking, we obtained 1,823 OTUs from 4 groups samples (Fig. 7). The sum of OTUs in Blank group (blank control group: 1,028) was higher than that of the other three groups (Vc-Dox treatment group: 323, CoQ10-Dox treatment group: 375, Dox treatment group: 800). Moreover, shared OTUs took up more than 24% between Vc-Dox treatment group and blank control group (248/1,028), CoQ10-Dox treatment group and blank control group (254/1,028). And shared OTUs took up about 21% between CoQ10-Dox treatment group and Dox treatment group (221/800), Vc-Dox treatment group and Dox treatment group (172/800). The number of OTUs shared by all four groups was 113. However, 635 species of bacteria were unique to the Dox treatment group (Dox), 28 species were unique to the CoQ10-Dox treatment group (C-Dox). This demonstrates that the Dox treatment group had produced a large number of new OTUs, and these bacteria are likely to be harmful.
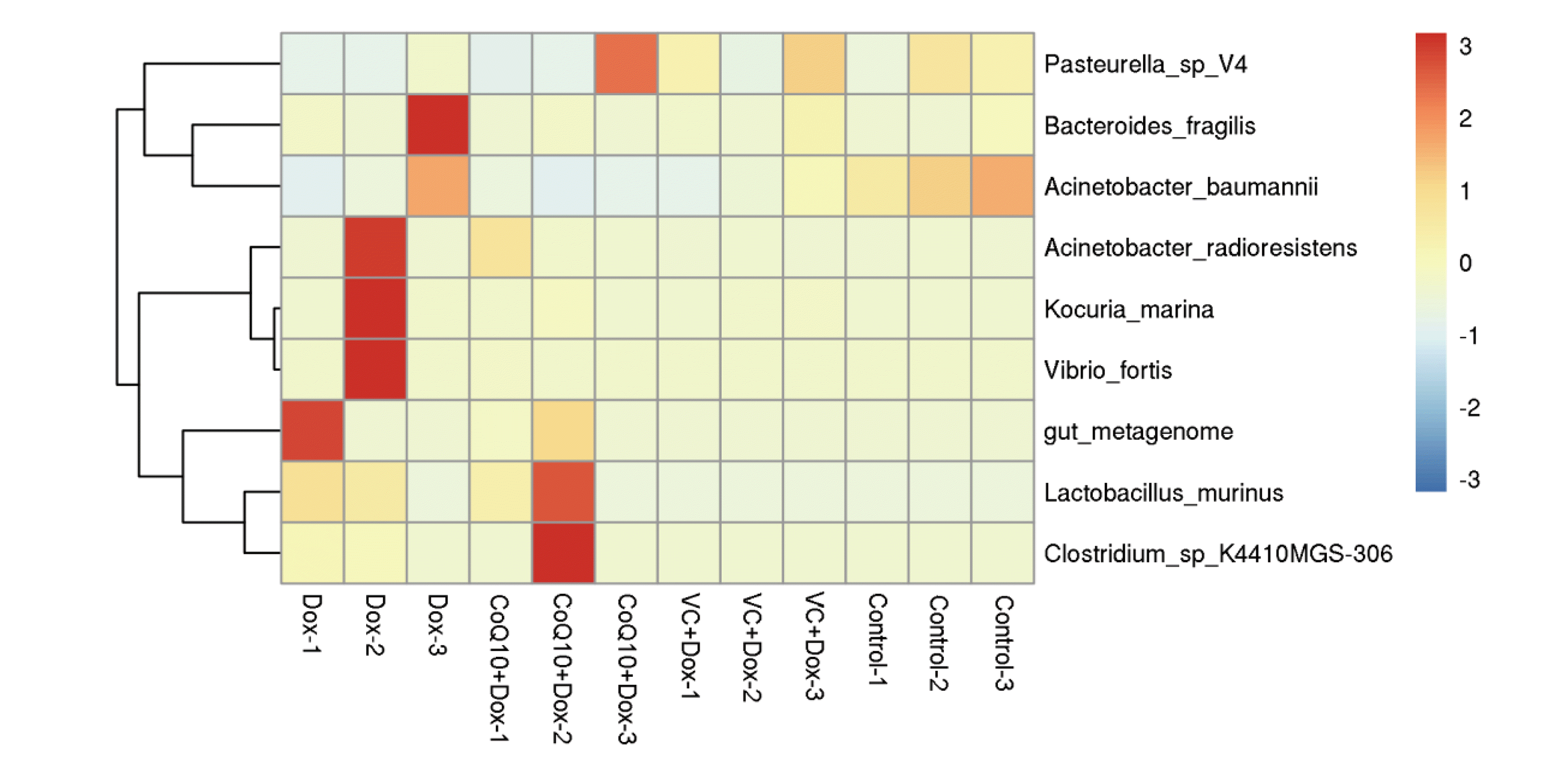
The detected microorganisms were classified from phylum to species at various taxonomic levels, and the results showed that there were significant differences among the intestinal flora of rats in each group. As shown in Fig. 8, the abundance of harmful bacterial groups Bacteroides fragilis, Acinetobacter radioresistens, Kocuria marina, and Vibrio fortis was significantly upregulated in the Dox treatment group compared with the normal control group. Compared with the Dox group, the VC + Dox group and the CoQ10 + Dox group had significantly reduced numbers of these harmful bacteria, while the abundance of Lactobacillus murinus significantly increased in the CoQ10 + Dox group. According to the above results, VC and CoQ10 treatment can reduce the damage of the intestinal flora by Dox treatment.
Then, we accounted the composition of the intestinal flora of each sample at each level of classification, and used the form of stacked graphs to intuitively show the variation of species abundance of different samples at each level of classification (Fig. 9). At the level of the phylum, the composition of various component of each sample shows that the number of Proteobacteria in the Blank group, CoQ10 + DOX group and Vc + DOX group has been significantly increased, and the number of Proteobacteria in the Dox sample has been significantly reduced, and the proportion of Bacteroidetes has been significantly increased, indicating that Dox affects the intestinal flora, while CoQ10 and VC may inhibit the increase in the number of intestinal flora such as Proteobacteria caused by Dox.
Comparing the species differences of intestinal flora between each group, It can be seen the differences between the Dox group and the CoQ10 + Dox group are significant (Fig. 10). The differences were mainly due to the increase of harmful bacteria such as Bacteroidetes, Bacteroidia, Bacteroidales and Prevotellaceae in the Dox group; however, beneficial strains such as Enterobacteriaceae, Gammaproteobacteria, Enterobacterales, Escherichia and Proteobacteria were reduced. It can be explained that Dox affects the intestinal flora and increases the harmful flora. The addition of CoQ10 protection can improve the intestinal flora and increase the beneficial intestinal bacteria, such as Enterobacteriaceae, Gammaproteobacteria, Enterobacterales, Escherichia and Proteobacteria. In the CoQ10 + Dox group, harmful strains such as Bacteroidetes, Bacteroidia, Bacteroidales, and Prevotellaceae were significantly reduced, which indicating that CoQ10 can improve the intestinal flora.
CoQ10 is a congenital antioxidant that has many different biological effects, such as enhancing immunity, free radical scavenging and DNA protection [8]. There is also evidence that CoQ10 deficiency is associated with certain cancers, such as breast cancer [9]. A survey showed that CoQ10 plays an important role in the development, prevention and treatment of breast cancer [10]. VC is a water-soluble antioxidant. Multiple experiments have indicated that VC can induce tumor apoptosis in a dose-dependent manner in vitro, and high-dose VC treatment can exert antitumor activity by increasing the amount of reactive oxygen species in cancer cells [11,12]. Therefore, high doses of intravenous VC are safe and can significantly slow tumor growth in animal experiments [13]. The efficacy and low toxicity to normal tissues are ideal expectations for cancer treatment, and the use of CoQ10 and VC as cancer treatment methods has far-reaching significance.
Dox is a broad-spectrum antitumor antibiotic with good antitumor activity, but its clinical application is greatly limited due to its strong side effects. Acute toxicity presents as nausea, vomiting, and arrhythmia; chronic toxicity is mainly manifested in the heart, liver, brain and kidney and other organs as damage. Mechanistically, DOX induces apoptosis or cell growth senescence through various molecular mechanisms, including inhibition of topoisomerase II, insertion of DNA and production of free radicals [14]. In this paper, an MTT assay and TUNEL staining was used to detect the proliferation capacity and apoptosis of GES-1 cells in different treatment groups. The results showed that Dox induced a significant decrease in GES-1 cell proliferation capacity and increase in apoptosis, and CoQ10 or VC pretreatment effectively inhibited Dox-induced damage in GES-1 cells. The levels of GSH and MDA in the gastric tissue of four groups of rats were detected by ELISA. The results showed that Dox stimulation caused a significant decrease in the GSH level in the gastric tissue and a significant increase in the MDA level. Therefore, the Dox-induced damage to the stomach may be caused by the excessive oxidation of gastric tissues caused by the generation of free radicals. Pretreatment with the antioxidants CoQ10 or VC significantly inhibited Dox-induced gastric mucosal damage.
NF-κB is a transcription factor that regulates the expression of several inflammatory mediators, such as TNF-α, IL-6 and IL-1β [15]. Pathological disorders of NF-κB signaling are associated with inflammation, associated autoimmune diseases, and the occurrence and development of cancer [16,17]. The NF-κB signaling pathway has both canonical and noncanonical pathways. The canonical IκB kinase (IKK) complex consists of catalytic IκBα and IκKβ subunits and a regulatory IKKγ subunit. Although both catalytic units of the IKK complex are capable of activating IκB, IκKβ plays a leading role in activating NF-κB signaling in response to inflammatory stimuli [18-20]. At the mRNA level, Dox stimulation resulted in a significant increase in the expression levels of IκKβ, IκBα, NF-κB/p65, and TNF-α in GES-1 cells. At the protein level, Dox treatment induced significantly increased expression of IκKβ, IκBα, nuclear NF-κB/p65 and TNF-α in GES-1 cells. The expression of NF-κB/p65 protein was significantly decreased in the cytoplasm, and pretreatment of GES-1 cells with VC or CoQ10 inhibited this effect of Dox. Therefore, CoQ10 or VC may inhibit Dox-induced gastric mucosal damage by inhibiting IkKB/IκBα/NF-κB/p65/TNF-α pathway activation.
It has been reported that serum TNF-α levels are increased in patients with heart failure. In rats with heart failure, elevated expression of TNF-α and IL-1β can synergistically induce cardiac systolic function decline and vascular endothelial function damage [21]. IL-6 is a multifunctional inflammatory factor, and several studies have shown that IL-6 plays an important role in the development and progression of cancer [22,23]. INF-γ is an anti-inflammatory mediator, and research has shown that ox-γ expression is elevated during cardiotoxicity induced by Dox [24]. One of the protective mechanisms of Sheng-Mai Yin on the myocardium is to inhibit Dox-induced myocardial injury by inhibiting the expression of TLR2, MCP-1, INF-γ and IL-6 to protect the myocardium [25]. The results of this study showed that the expression of TNF-α, IL-6 and INF-γ in the gastric tissue and serum of the Dox group was significantly increased. Dox-induced expression of TNF-α, IL-6 and INF-γ was significantly inhibited by CoQ10 or VC treatment. Therefore, CoQ10 or VC treatment inhibits Dox-induced gastric mucosal damage by regulating the anti-inflammatory action of the gastric tissue.
The gut flora and intestinal mucosa together form a protective barrier that prevents the entry of bacteria, viruses and food antigens. There are more than 400 types of flora and 14 genera in the human intestine, which together constitute the microecological environment of the human intestine. Bacteroides fragilis is an obligate anaerobic, gram-negative rod-shaped bacterium. It is usually symbiotic and a common opportunistic pathogen in clinical infections. In human colons, infections caused by Bacteroides fragilis account for 60%–90% of all anaerobic infections [26]. Acinetobacter radioresistens is one of the genera of Acinetobacter. When the immune system is compromised, it is easier for pathogens to cause infection, and Acinetobacter is one of the important opportunistic pathogens causing infection in hospitals. Kocuria marina, a member of the Micrococcaceae, is often found in the environment and on human skin, and studies have reported that the bacteria can cause human peritonitis [27]. It has been reported that Vibrio fortis can cause hippocampal enteritis [28]. 16S rDNA sequencing results showed that, compared with the no treatment control, Dox treatment caused intestinal flora disorder, resulting in the increase of the harmful flora Bacteroides fragilis, Acinetobacter radioresistens, Kocuria marina and Vibrio fortis. CoQ10 or VC treatment inhibited the harmful effects of Dox compared with Dox treatment alone. Probiotics and their metabolites can induce interferon, increase cytokinins, activate immune cells, promote the production of immunoglobulins, improve immunity and inhibit tumorigenesis, and can also treat allergies such as food allergies, eczema and allergic dermatitis reactive disease. Studies have reported that probiotics can improve and protect intestinal mucosal function [29]. The 16S rDNA sequencing results showed that CoQ10 treatment caused an increase in the number of probiotic Lactobacillus murinus in the intestine compared with the Dox treatment alone. Based on the above results, we conclude that CoQ10 and VC treatment can improve the intestinal flora disturbance caused by Dox treatment and protect intestinal health.
Supplementary data including three figures can be found with this article online at https://doi.org/10.4196/kjpp.2021.25.4.261.
ACKNOWLEDGEMENTS
This work was financially supported by the National Natural Science Foundation of China (No. 21878220).
Notes
REFERENCES
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. 2018; Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 68:394–424. DOI: 10.3322/caac.21492. PMID: 30207593.

2. Heart EA, Karandrea S, Liang X, Balke ME, Beringer PA, Bobczynski EM, Zayas-Bazán Burgos D, Richardson T, Gray JP. 2016; Mechanisms of doxorubicin toxicity in pancreatic β-cells. Toxicol Sci. 152:395–405. DOI: 10.1093/toxsci/kfw096. PMID: 27255381. PMCID: PMC4960911.

3. Zhao Y, Huan ML, Liu M, Cheng Y, Sun Y, Cui H, Liu DZ, Mei QB, Zhou SY. 2016; Doxorubicin and resveratrol co-delivery nanoparticle to overcome doxorubicin resistance. Sci Rep. 6:35267. DOI: 10.1038/srep35267. PMID: 27731405. PMCID: PMC5059704.

4. Rivankar S. 2014; An overview of doxorubicin formulations in cancer therapy. J Cancer Res Ther. 10:853–858. DOI: 10.4103/0973-1482.139267. PMID: 25579518.

5. Eng MS, Kaur J, Prasmickaite L, Engesæter BØ, Weyergang A, Skarpen E, Berg K, Rosenblum MG, Mælandsmo GM, Høgset A, Ferrone S, Selbo PK. 2018; Enhanced targeting of triple-negative breast carcinoma and malignant melanoma by photochemical internalization of CSPG4-targeting immunotoxins. Photochem Photobiol Sci. 17:539–551. DOI: 10.1039/C7PP00358G. PMID: 29565434.

6. Frontiñán-Rubio J, Santiago-Mora RM, Nieva-Velasco CM, Ferrín G, Martínez-González A, Gómez MV, Moreno M, Ariza J, Lozano E, Arjona-Gutiérrez J, Gil-Agudo A, De la Mata M, Pesic M, Peinado JR, Villalba JM, Pérez-Romasanta L, Pérez-García VM, Alcaín FJ, Durán-Prado M. 2018; Regulation of the oxidative balance with coenzyme Q10 sensitizes human glioblastoma cells to radiation and temozolomide. Radiother Oncol. 128:236–244. DOI: 10.1016/j.radonc.2018.04.033. PMID: 29784452.

7. Terán E, Racines-Orbe M, Toapanta J, Valdivieso L, Vega Z, Vivero S, Moya W, Chedraui P, Pérez-López FR. 2011; Maternal plasma and amniotic fluid coenzyme Q10 levels in preterm and term gestations: a pilot study. Arch Gynecol Obstet. 283 Suppl 1:67–71. DOI: 10.1007/s00404-011-1894-x. PMID: 21547699.

8. Bahar M, Khaghani S, Pasalar P, Paknejad M, Khorramizadeh MR, Mirmiranpour H, Nejad SG. 2010; Exogenous coenzyme Q10 modulates MMP-2 activity in MCF-7 cell line as a breast cancer cellular model. Nutr J. 9:62. DOI: 10.1186/1475-2891-9-62. PMID: 21118526. PMCID: PMC3004807.

9. Cooney RV, Dai Q, Gao YT, Chow WH, Franke AA, Shu XO, Li H, Ji B, Cai Q, Chai W, Zheng W. 2011; Low plasma coenzyme Q(10) levels and breast cancer risk in Chinese women. Cancer Epidemiol Biomarkers Prev. 20:1124–1130. DOI: 10.1158/1055-9965.EPI-10-1261. PMID: 21467235. PMCID: PMC3545677.

10. Tafazoli A. 2017; Coenzyme Q10 in breast cancer care. Future Oncol. 13:1035–1041. DOI: 10.2217/fon-2016-0547. PMID: 28481148.

11. Lee SJ, Jeong JH, Lee IH, Lee J, Jung JH, Park HY, Lee DH, Chae YS. 2019; Effect of high-dose vitamin C combined with anti-cancer treatment on breast cancer cells. Anticancer Res. 39:751–758. DOI: 10.21873/anticanres.13172. PMID: 30711954.

12. Nagappan A, Park KI, Park HS, Kim JA, Hong GE, Kang SR, Lee DH, Kim EH, Lee WS, Won CK, Kim GS. 2012; Vitamin C induces apoptosis in AGS cells by down-regulation of 14-3-3σ via a mitochondrial dependent pathway. Food Chem. 135:1920–1928. DOI: 10.1016/j.foodchem.2012.06.050. PMID: 22953941.

13. Yang G, Yan Y, Ma Y, Yang Y. 2017; Vitamin C at high concentrations induces cytotoxicity in malignant melanoma but promotes tumor growth at low concentrations. Mol Carcinog. 56:1965–1976. DOI: 10.1002/mc.22654. PMID: 28370562.

14. Meredith AM, Dass CR. 2016; Increasing role of the cancer chemotherapeutic doxorubicin in cellular metabolism. J Pharm Pharmacol. 68:729–741. DOI: 10.1111/jphp.12539. PMID: 26989862.

15. Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN, Timmers L, van Rijen HV, Doevendans PA, Pasterkamp G, Lim SK, de Kleijn DP. 2013; Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 10:301–312. DOI: 10.1016/j.scr.2013.01.002. PMID: 23399448.

16. Hoesel B, Schmid JA. 2013; The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. 12:86. DOI: 10.1186/1476-4598-12-86. PMID: 23915189. PMCID: PMC3750319.

17. Sun SC, Chang JH, Jin J. 2013; Regulation of nuclear factor-κB in autoimmunity. Trends Immunol. 34:282–289. DOI: 10.1016/j.it.2013.01.004. PMID: 23434408. PMCID: PMC3664242.

18. Tak PP, Firestein GS. 2001; NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 107:7–11. DOI: 10.1172/JCI11830. PMID: 11134171. PMCID: PMC198552.
19. Leung CH, Chan DS, Li YW, Fong WF, Ma DL. 2013; Hit identification of IKKβ natural product inhibitor. BMC Pharmacol Toxicol. 14:3. DOI: 10.1186/2050-6511-14-3. PMID: 23294515. PMCID: PMC3583241.

20. Patil KR, Mohapatra P, Patel HM, Goyal SN, Ojha S, Kundu CN, Patil CR. 2015; Pentacyclic triterpenoids inhibit IKKβ mediated activation of NF-κB pathway: in silico and in vitro evidences. PLoS One. 10:e0125709. DOI: 10.1371/journal.pone.0125709. PMID: 25938234. PMCID: PMC4418667.

21. Kleinbongard P, Heusch G, Schulz R. 2010; TNFalpha in atherosclerosis, myocardial ischemia/reperfusion and heart failure. Pharmacol Ther. 127:295–314. DOI: 10.1016/j.pharmthera.2010.05.002. PMID: 20621692.
22. Fu ZZ, Peng Y, Cao LY, Chen YS, Li K, Fu BH. 2016; Correlations between serum IL-6 levels and radiation pneumonitis in lung cancer patients: a meta-analysis. J Clin Lab Anal. 30:145–154. DOI: 10.1002/jcla.21828. PMID: 25545734. PMCID: PMC6806722.

23. Li J, Lan T, Zhang C, Zeng C, Hou J, Yang Z, Zhang M, Liu J, Liu B. 2015; Reciprocal activation between IL-6/STAT3 and NOX4/Akt signalings promotes proliferation and survival of non-small cell lung cancer cells. Oncotarget. 6:1031–1048. DOI: 10.18632/oncotarget.2671. PMID: 25504436. PMCID: PMC4359215.

24. Horie T, Ono K, Nishi H, Nagao K, Kinoshita M, Watanabe S, Kuwabara Y, Nakashima Y, Takanabe-Mori R, Nishi E, Hasegawa K, Kita T, Kimura T. 2010; Acute doxorubicin cardiotoxicity is associated with miR-146a-induced inhibition of the neuregulin-ErbB pathway. Cardiovasc Res. 87:656–664. DOI: 10.1093/cvr/cvq148. PMID: 20495188. PMCID: PMC2920811.

25. Ma S, Li X, Dong L, Zhu J, Zhang H, Jia Y. 2016; Protective effect of Sheng-Mai Yin, a traditional Chinese preparation, against doxorubicin-induced cardiac toxicity in rats. BMC Complement Altern Med. 16:61. DOI: 10.1186/s12906-016-1037-9. PMID: 26865364. PMCID: PMC4750239.

26. Rocha ER, Smith CJ. 2013; Ferritin-like family proteins in the anaerobe Bacteroides fragilis: when an oxygen storm is coming, take your iron to the shelter. Biometals. 26:577–591. DOI: 10.1007/s10534-013-9650-2. PMID: 23842847. PMCID: PMC3815548.

27. Lee JY, Kim SH, Jeong HS, Oh SH, Kim HR, Kim YH, Lee JN, Kook JK, Kho WG, Bae IK, Shin JH. 2009; Two cases of peritonitis caused by Kocuria marina in patients undergoing continuous ambulatory peritoneal dialysis. J Clin Microbiol. 47:3376–3378. DOI: 10.1128/JCM.00847-09. PMID: 19692561. PMCID: PMC2756933.
28. Wang X, Zhang Y, Qin G, Luo W, Lin Q. 2016; A novel pathogenic bacteria (Vibrio fortis) causing enteritis in cultured seahorses, Hippocampus erectus Perry, 1810. J Fish Dis. 39:765–769. DOI: 10.1111/jfd.12411. PMID: 26466548.
29. Guarino A, Ashkenazi S, Gendrel D, Lo Vecchio A, Shamir R, Szajewska H. 2014; European Society for Pediatric Gastroenterology, Hepatology, and Nutrition/European Society for Pediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe: update 2014. J Pediatr Gastroenterol Nutr. 59:132–152. DOI: 10.1097/MPG.0000000000000375. PMID: 24739189.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download