1. Siegel R, Ma J, Zou Z, Jemal A. 2014; Cancer statistics, 2014. CA Cancer J Clin. 64:9–29. DOI:
10.3322/caac.21208. PMID:
24399786.

4. Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, DeForge L, Koumenis IL, Lewis D, Harris L, Bussiere J, Koeppen H, Shahrokh Z, Schwall RH. 1999; Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 104:155–162. DOI:
10.1172/JCI6926. PMID:
10411544. PMCID:
PMC408479.

5. Sheridan JP, Marsters SA, Pitti RM, Gurney A, Skubatch M, Baldwin D, Ramakrishnan L, Gray CL, Baker K, Wood WI, Goddard AD, Godowski P, Ashkenazi A. 1997; Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science. 277:818–821. DOI:
10.1126/science.277.5327.818. PMID:
9242611.

6. Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM. 1997; An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science. 277:815–818. DOI:
10.1126/science.277.5327.815. PMID:
9242610.

7. Emery JG, McDonnell P, Burke MB, Deen KC, Lyn S, Silverman C, Dul E, Appelbaum ER, Eichman C, DiPrinzio R, Dodds RA, James IE, Rosenberg M, Lee JC, Young PR. 1998; Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J Biol Chem. 273:14363–14367. DOI:
10.1074/jbc.273.23.14363. PMID:
9603945.

9. Zhang L, Fang B. 2005; Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther. 12:228–237. DOI:
10.1038/sj.cgt.7700792. PMID:
15550937.

10. Sanlioglu AD, Dirice E, Aydin C, Erin N, Koksoy S, Sanlioglu S. 2005; Surface TRAIL decoy receptor-4 expression is correlated with TRAIL resistance in MCF7 breast cancer cells. BMC Cancer. 5:54. DOI:
10.1186/1471-2407-5-54. PMID:
15916713. PMCID:
PMC1156874.

11. Griffith TS, Kucaba TA, O'Donnell MA, Burns J, Benetatos C, McKinlay MA, Condon S, Chunduru S. 2011; Sensitization of human bladder tumor cells to TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis with a small molecule IAP antagonist. Apoptosis. 16:13–26. DOI:
10.1007/s10495-010-0535-3. PMID:
20734142.

13. King AA, Shaughnessy DT, Mure K, Leszczynska J, Ward WO, Umbach DM, Xu Z, Ducharme D, Taylor JA, Demarini DM, Klein CB. 2007; Antimutagenicity of cinnamaldehyde and vanillin in human cells: global gene expression and possible role of DNA damage and repair. Mutat Res. 616:60–69. DOI:
10.1016/j.mrfmmm.2006.11.022. PMID:
17178418. PMCID:
PMC1955325.

14. inivasan K Sr, Platel K, Rao MVL. 2008; Hypotriglyceridemic effect of dietary vanillin in experimental rats. Eur Food Res Technol. 228:103–108. DOI:
10.1007/s00217-008-0911-1.

15. Imanishi H, Sasaki YF, Matsumoto K, Watanabe M, Ohta T, Shirasu Y, Tutikawa K. 1990; Suppression of 6-TG-resistant mutations in V79 cells and recessive spot formations in mice by vanillin. Mutat Res. 243:151–158. DOI:
10.1016/0165-7992(90)90038-L. PMID:
2304483.

16. Liang JA, Wu SL, Lo HY, Hsiang CY, Ho TY. 2009; Vanillin inhibits matrix metalloproteinase-9 expression through down-regulation of nuclear factor-kappaB signaling pathway in human hepatocellular carcinoma cells. Mol Pharmacol. 75:151–157. DOI:
10.1124/mol.108.049502. PMID:
18835982.
17. Lirdprapamongkol K, Sakurai H, Suzuki S, Koizumi K, Prangsaengtong O, Viriyaroj A, Ruchirawat S, Svasti J, Saiki I. 2010; Vanillin enhances TRAIL-induced apoptosis in cancer cells through inhibition of NF-kappaB activation. In Vivo. 24:501–506. PMID:
20668316.
18. Lirdprapamongkol K, Sakurai H, Kawasaki N, Choo MK, Saitoh Y, Aozuka Y, Singhirunnusorn P, Ruchirawat S, Svasti J, Saiki I. 2005; Vanillin suppresses in vitro invasion and in vivo metastasis of mouse breast cancer cells. Eur J Pharm Sci. 25:57–65. DOI:
10.1016/j.ejps.2005.01.015. PMID:
15854801.

19. Lirdprapamongkol K, Kramb JP, Suthiphongchai T, Surarit R, isomsap C Sr, Dannhardt G, Svasti J. 2009; Vanillin suppresses metastatic potential of human cancer cells through PI3K inhibition and decreases angiogenesis in vivo. J Agric Food Chem. 57:3055–3063. DOI:
10.1021/jf803366f. PMID:
19368348.

20. Yadav VR, Sung B, Prasad S, Kannappan R, Cho SG, Liu M, Chaturvedi MM, Aggarwal BB. 2010; Celastrol suppresses invasion of colon and pancreatic cancer cells through the downregulation of expression of CXCR4 chemokine receptor. J Mol Med. 88:1243–1253. DOI:
10.1007/s00109-010-0669-3. PMID:
20798912. PMCID:
PMC3142743.

21. Tsuda H, Uehara N, Iwahori Y, Asamoto M, Iigo M, Nagao M, Matsumoto K, Ito M, Hirono I. 1994; Chemopreventive effects of beta-carotene, alpha-tocopherol and five naturally occurring antioxidants on initiation of hepatocarcinogenesis by 2-amino-3-methylimidazo [4,5-f]quinoline in the rat. Jpn J Cancer Res. 85:1214–1219. DOI:
10.1111/j.1349-7006.1994.tb02932.x. PMID:
7852184. PMCID:
PMC5919387.
23. Gustafson DL, Franz HR, Ueno AM, Smith CJ, Doolittle DJ, Waldren CA. 2000; Vanillin (3-methoxy-4-hydroxybenzaldehyde) inhibits mutation induced by hydrogen peroxide, N-methyl-N-nitrosoguanidine and mitomycin C but not (137)Cs gamma-radiation at the CD59 locus in human-hamster hybrid A(L) cells. Mutagenesis. 15:207–213. DOI:
10.1093/mutage/15.3.207. PMID:
10792012.

24. Chawla-Sarkar M, Bae SI, Reu FJ, Jacobs BS, Lindner DJ, Borden EC. 2004; Downregulation of Bcl-2, FLIP or IAPs (XIAP and survivin) by siRNAs sensitizes resistant melanoma cells to Apo2L/TRAIL-induced apoptosis. Cell Death Differ. 11:915–923. DOI:
10.1038/sj.cdd.4401416. PMID:
15118763.

25. Deveraux QL, Takahashi R, Salvesen GS, Reed JC. 1997; X-linked IAP is a direct inhibitor of cell-death proteases. Nature. 388:300–304. DOI:
10.1038/40901. PMID:
9230442.

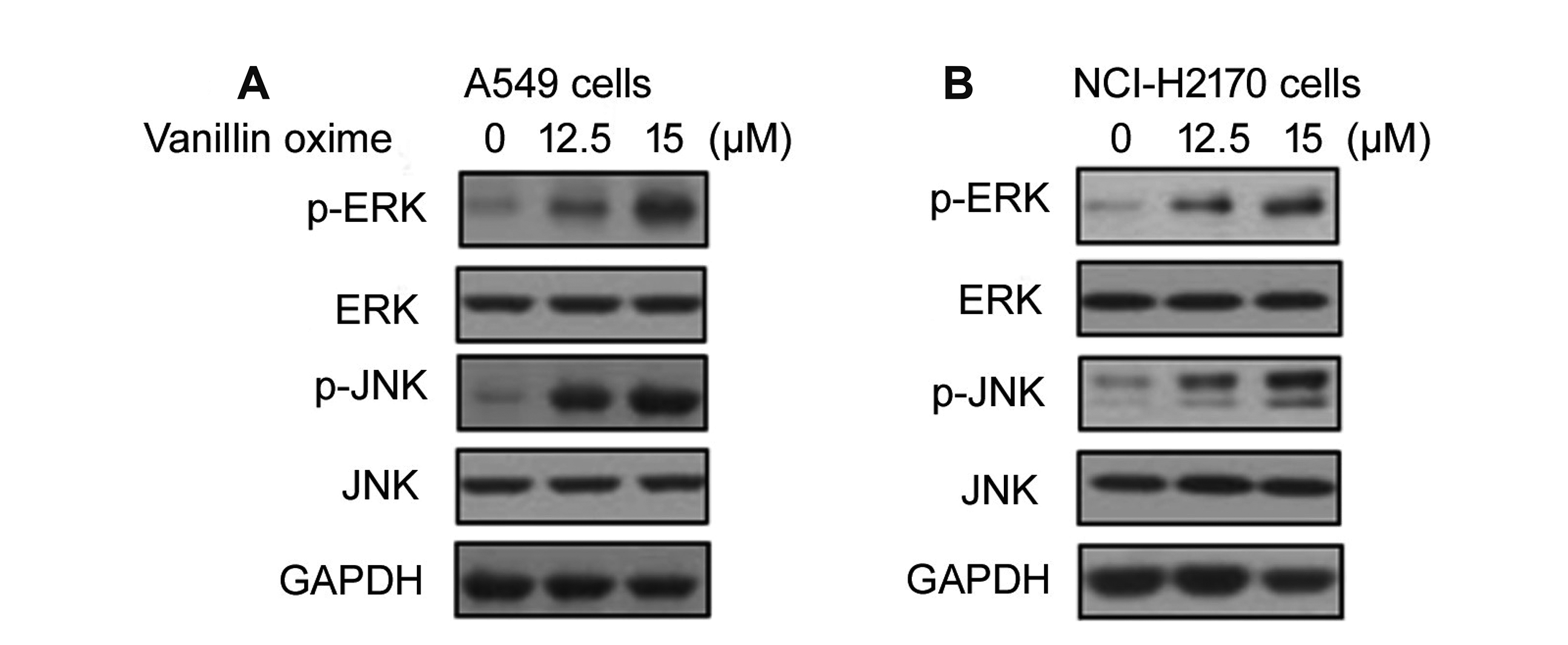
26. Gupta SC, Reuter S, Phromnoi K, Park B, Hema PS, Nair M, Aggarwal BB. 2011; Nimbolide sensitizes human colon cancer cells to TRAIL through reactive oxygen species- and ERK-dependent up-regulation of death receptors, p53, and Bax. J Biol Chem. 286:1134–1146. Retraction. DOI:
10.1074/jbc.M110.191379. PMID:
21078664. PMCID:
PMC3020720.

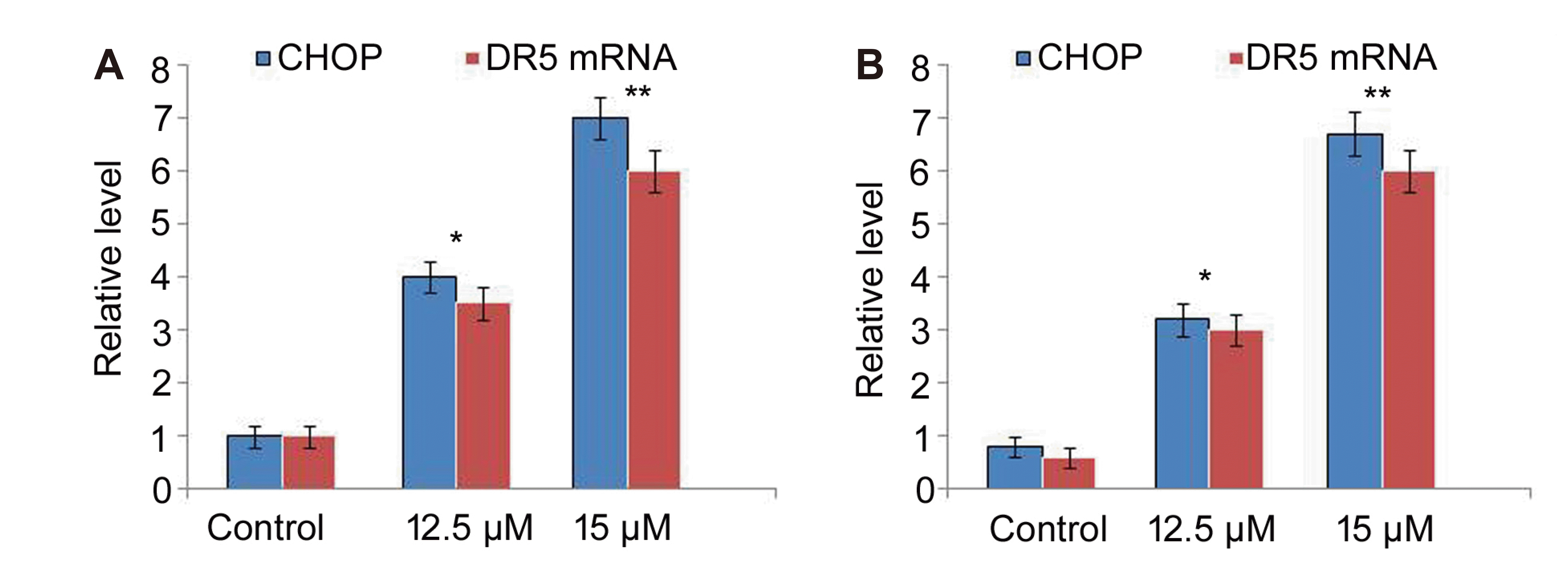
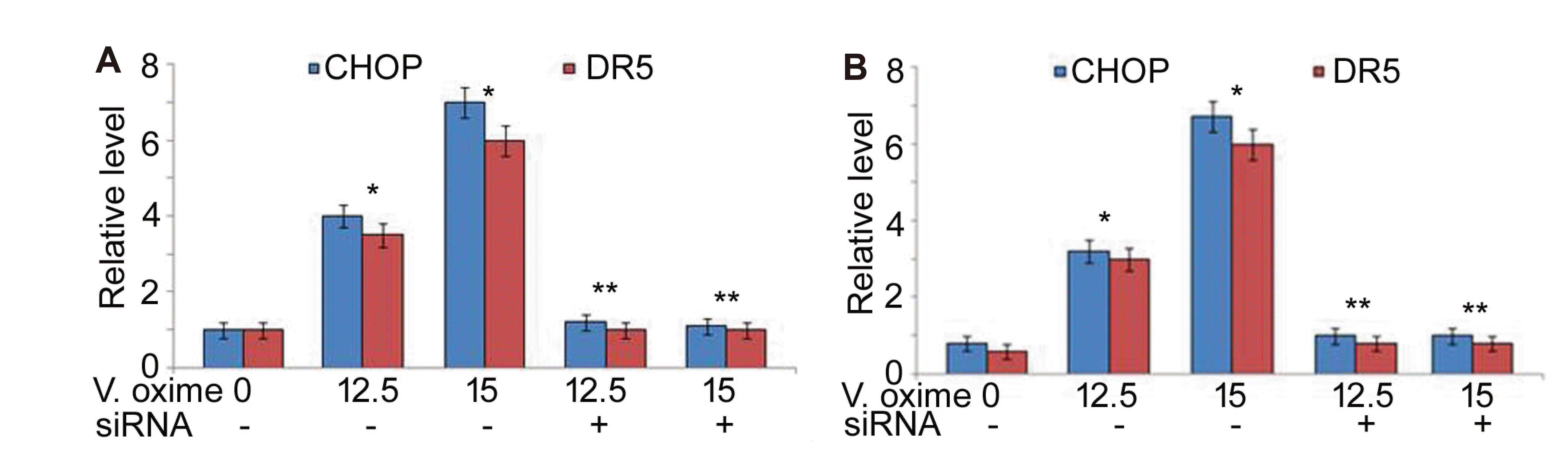
27. Moon DO, Park SY, Choi YH, Ahn JS, Kim GY. 2011; Guggulsterone sensitizes hepatoma cells to TRAIL-induced apoptosis through the induction of CHOP-dependent DR5: involvement of ROS-dependent ER-stress. Biochem Pharmacol. 82:1641–1650. DOI:
10.1016/j.bcp.2011.08.019. PMID:
21903093.

28. Fisher MJ, Virmani AK, Wu L, Aplenc R, Harper JC, Powell SM, Rebbeck TR, Sidransky D, Gazdar AF, El-Deiry WS. 2001; Nucleotide substitution in the ectodomain of trail receptor DR4 is associated with lung cancer and head and neck cancer. Clin Cancer Res. 7:1688–1697. PMID:
11410508.
30. Lin N, Sato T, Takayama Y, Mimaki Y, Sashida Y, Yano M, Ito A. 2003; Novel anti-inflammatory actions of nobiletin, a citrus polymethoxy flavonoid, on human synovial fibroblasts and mouse macrophages. Biochem Pharmacol. 65:2065–2071. DOI:
10.1016/S0006-2952(03)00203-X. PMID:
12787887.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download