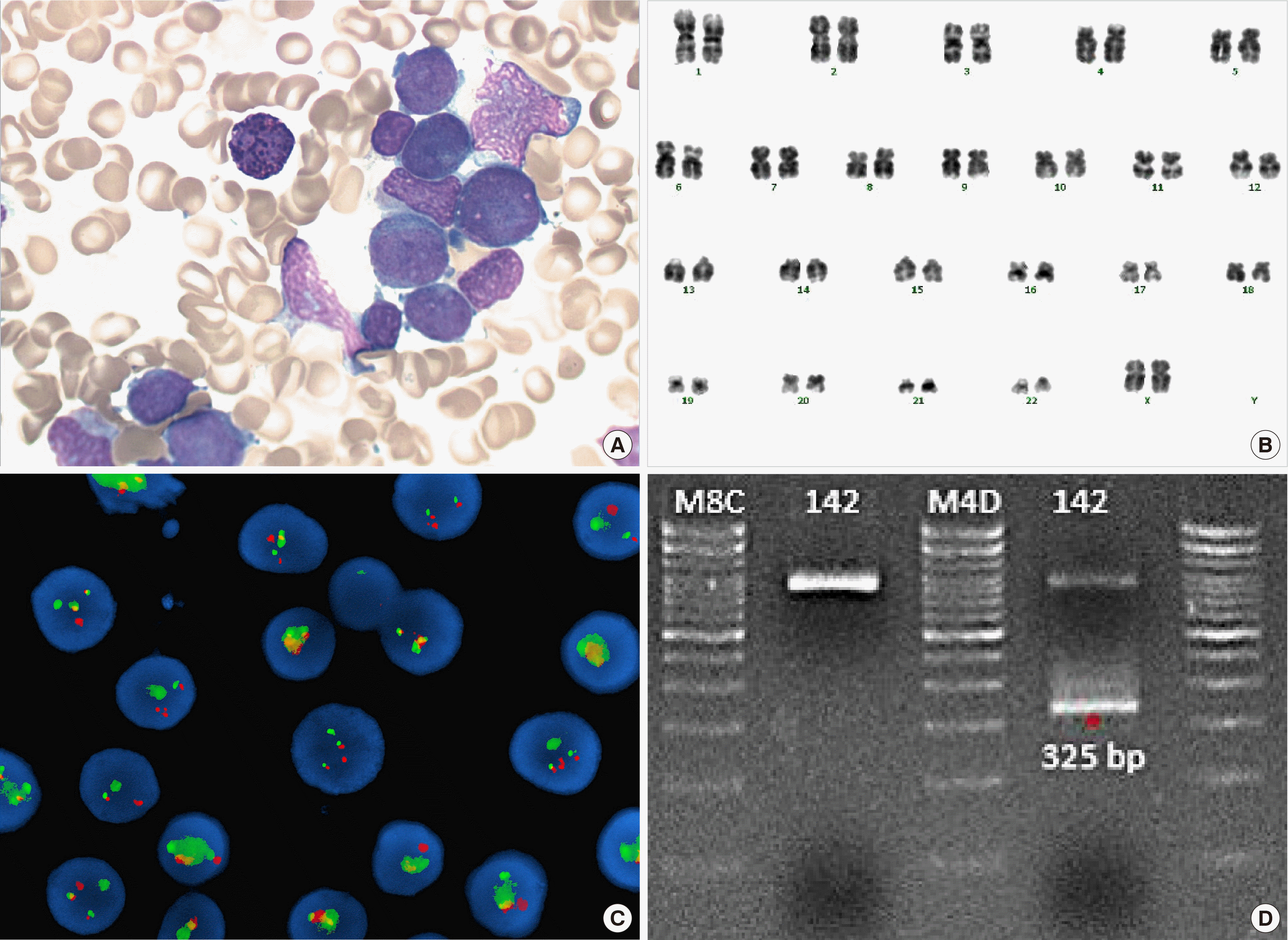
 | Fig. 1(A) Granular promyelocytes from bone marrow aspiration smear (Wright-Giemsa stain, ×1,000). (B) Giemsa-banding karyotyping of the bone marrow cells at diagnosis reveals the karyotype 46,XX. (C) Fluorescence in situ hybridization (FISH) study using a PML/RARA dual-color, dual-fusion translocation probe at diagnosis. Fusion signals were observed. (D) RT-PCR for PML/RARA fusion transcripts. S-form (325 bp) PML/RARA chimeric transcripts were amplified.
Abbreviation: RT-PCR, reverse-transcriptase polymerase chain reaction.
|
Dear Editor,
Acute promyelocytic leukemia (APL) comprises 5−8% of acute myeloid leukemia (AML) [1]. The t(15;17)(q22;q12) with PML-RARA fusion transcript is reported in 90–95% of APL cases, while other structural rearrangements, including submicroscopic translocation, have been noted in the remaining cases [2]. Therapy-related myeloid neoplasms (t-MNs) comprising therapy-related AML, myelodysplastic syndrome (MDS), and myelodysplastic/myeloproliferative neoplasms (MDS/MPNs) account for 10−20% of all cases of AML, MDS, and MDS/MPNs. Approximately 8–21% APL cases are therapy-related APL (t-APL), and cryptic PML-RARA rearrangement in t-APL has been rarely reported [3, 4]. Here, we report a case of cryptic t-APL patient.
A 66-year-old female patient was admitted to hospital for oral bleeding. In 2016, she was diagnosed with triple-negative breast cancer, left (stage 3, pT2N3M0, P53: 90%, Ki-67: 60%, estrogen receptor: negative, progesterone receptor: negative, human epidermal growth factor receptor 2: negative) and underwent breast conserving surgery with axillary lymph node dissection. After surgery, the patient was treated with six cycles of adjuvant chemotherapy (docetaxel, doxorubicin, and cyclophosphamide) for 3 months with radiation therapy.
The result of initial complete blood count revealed a hemoglobin level of 9.7 g/dL, white blood cell (WBC) count of 48.2×109/L (segmented neutrophils, 5%; band form neutrophils, 0%; lymphocytes, 3%; monocytes, 2%; eosinophils, 1%; and blast, 86%), and platelet count of 48×109/L. The bone marrow aspirate was filled with morphologically abnormal myeloblasts/promyelocytes, which accounted for 93% of all nucleated cells. These cells had azurophilic granulation and ovoid nuclei; a few of them had Auer rods (Fig. 1A). Bone marrow biopsy demonstrated a hypercellular marrow approaching 95% cellularity. Flow cytometry analysis showed that the blasts were positive for CD13 (bright); CD33, CD117, CD2, CD56 (intermediate); CD54, CD41, and CD7 (dim); and negative for CD34, TdT, HLA-DR, CD15, CD14, CD19, CD19, cCD22, CD3, cCD3, and myeloperoxidase (MPO). The karyotype of this patient was 46,XX in all 20 metaphase cells analyzed (Fig. 1B). Fluorescence in situ hybridization (FISH) analysis using a dual-color dual-fusion PML/RARA probe revealed two fusion signals in 496 out of 500 interphase cells; nuc ish (PML,RARA)×3 (RARA con PML×2)[496/500] (Fig. 1C). Next-generation-sequencing screening for 49 genes related to AML identified internal tandem repeats of the FLT3 gene (FLT3-ITD); the variant allele frequency (VAF) was 39% at diagnosis, as confirmed by fragment analysis. Reverse-transcriptase polymerase chain reaction (RT-PCR) for PML-RARA rearrangement showed positive result for a fusion transcript (short form, bcr3) (Fig. 1D). Therefore, the patient was diagnosed with t-APL. Treatment with all-trans-retinoic acid (ATRA) was initiated. On the 6th day of admission, pulmonary hemorrhage and pneumonia worsened, which led to pneumonic septic shock and multi-organ failure. The patient died at day 35 after hospitalization.
To reduce life-threatening complications such as disseminated intravascular coagulation and improve treatment results, accurate and rapid diagnosis of APL is imperative and arsenic trioxide and ATRA and arsenic trioxide (ATO)-based therapies must be initiated. Chromosome analysis can detect PML-RARA fusion in about 90% of APL cases but not in cryptic APL [5], necessitating FISH or PCR to detect the abnormality of the fusion transcript. Further, APL that is PML-RAR negative in FISH is rare but does exist [6]. It seems important to perform RT-PCR test as well as chromosome analysis and FISH for clinical assessment of PML-RARA.
Mutations involving FLT3, including internal tandem duplication (ITD) and tyrosine kinase domain (TKD) mutations, occur in 35−40% of APL. The FLT3-ITD mutation is the most common and associated with higher WBC counts, microgranular granulocytes, and bcr3 breakpoint in PML [7].
The difference in survival outcomes in de novo APL or t-APL patients is controversial. In a previous study, the outcome of t-APL was similar to that of de novo APL [4]. Nevertheless, for all subtypes of AML, clinical outcomes were worse for patients with therapy-related disease [8]. Elevated WBC counts predict worse prognosis, but the FLT3-ITD mutation of APL has no significant effect on prognosis. However, a higher FLT3-ITD mutation/wild-type ratio (≥0.5%) indicated adverse prognosis [9].
To our knowledge, this is the first report of cryptic t-APL in Korea. Further study is warranted to estimate the prevalence of cryptic t-APL and to evaluate response to treatment and prognosis in t-APL patients.
Go to : 
REFERENCES
1. Swerdlow SH, Campo E, editors. 2017. WHO classification of tumours of haematopoietic and lymphoid tissues. Revised 4th edition. International Agency for Research on Cancer;Lyon, France: p. 134–55.
2. Burns TF, Loo EY, Bengtson EM, Bao L. 2019; Cytogenetically cryptic insertion of PML segment into RARA on chromosome 17q resulting PML-RARA fusion in acute promyelocytic leukemia. Ann Hematol. 98:211–3. DOI: 10.1007/s00277-018-3399-1. PMID: 30030569.

3. Kayser S, Krzykalla J, Elliott MA, Norsworthy K, Gonzales P, Hills RK, et al. 2017; Characteristics and outcome of patients with therapy-related acute promyelocytic leukemia front-line treated with or without arsenic trioxide. Leukemia. 31:2347–54. DOI: 10.1038/leu.2017.92. PMID: 28322237. PMCID: PMC6037311.

4. Beaumont M, Sanz M, Carli PM, Maloisel F, Thomas X, Detourmignies L, et al. 2003; Therapy-related acute promyelocytic leukemia. J Clin Oncol. 21:2123–37. DOI: 10.1200/JCO.2003.09.072. PMID: 12775738.

5. Kim KE, Woo KS, Kim SH, Han JY. 2009; Detection of PML/RARA rearrangement by reverse transcriptase-PCR and sequencing in a case of microgranular acute promyelocytic leukemia lacking t(15;17) on karyotype and FISH. Korean J Lab Med. 29:379–83. DOI: 10.3343/kjlm.2009.29.5.379. PMID: 19893344.

6. Kim MJ, Cho SY, Kim MH, Lee JJ, Kang SY, Cho EH, et al. 2010; FISH-negative cryptic PML-RARA rearrangement detected by long-distance polymerase chain reaction and sequencing analyses: a case study and review of the literature. Cancer Genet Cytogenet. 203:278–83. DOI: 10.1016/j.cancergencyto.2010.08.026. PMID: 21156244.

7. Breccia M, Loglisci G, Loglisci MG, Ricci R, Diverio D, Latagliata R, et al. 2013; FLT3-ITD confers poor prognosis in patients with acute promyelocytic leukemia treated with AIDA protocols: long-term follow-up analysis. Haematologica. 98:e161–3. DOI: 10.3324/haematol.2013.095380. PMID: 24323990. PMCID: PMC3856980.

8. Kayser S, Döhner K, Krauter J, Köhne CH, Horst HA, Held G, et al. 2011; The impact of therapy-related acute myeloid leukemia (AML) on outcome in 2853 adult patients with newly diagnosed AML. Blood. 117:2137–45. DOI: 10.1182/blood-2010-08-301713. PMID: 21127174.

9. Schnittger S, Bacher U, Haferlach C, Kern W, Alpermann T, Haferlach T. 2011; Clinical impact of FLT3 mutation load in acute promyelocytic leukemia with t(15;17)/PML-RARA. Haematologica. 96:1799–807. DOI: 10.3324/haematol.2011.049007. PMID: 21859732. PMCID: PMC3232262.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download