Abstract
Sarcoidosis can involve multiple organs and is characterized by non-caseating granuloma on biopsy, which is not pathognomonic of this disease. Therefore, diagnosis requires exclusion of other causes of granuloma, including malignant neoplasm, infections, autoimmune diseases, drug exposure, environmental causes, lymphoma, and tuberculosis. Herein, we present a rare case of a patient with a primary manifestation of sarcoidosis, with typical bilateral hilar adenopathy, and recurrence in the bone marrow. A 50-year-old female patient, who had been diagnosed with sarcoidosis at age 38, was admitted for petechia on both legs and pancytopenia. The patient was concluded to be “highly probable” for sarcoidosis, with at least 90% likelihood, according to the World Association of Sarcoidosis and Other Granulomatous Diseases (WASOG) organ assessment instrument. Moreover, the patient had a bone marrow biopsy positive for granulomatous inflammation. Hence, all findings supported the diagnosis of sarcoidosis. Because sarcoidosis is difficult to definitively diagnose, it will be useful to better understand the application, interpretation, and limitations of the WASOG instrument for bone marrow involvement assessment.
초록
유육종증(sarcoidosis)은 인체의 모든 장기를 침범할 수 있으며 조직학적으로 비건락성 육아종(non-caseating granuloma)을 특징으로 하는 질환이다. 유육종증 환자에서 골수침범은 30-50% 정도에서 있는 것으로 알려져 있으나 범혈구감소증 등의 증후를 나타내어 이것으로 골수생검을 통해 진단에 이르는 경우는 매우 드물다. 유육종증이 골수를 침범하는 경우 몇 가지 특징적인 임상양상을 나타내는데, 말초혈액에서는 빈혈, 백혈구감소증 등을 나타내며, 골수에서 육아종성 병변을 보이는 경우 감별해야 할 질환으로 유육종증 외에도 악성질환, 감염증, 자가면역질환, 약물 노출, 환경관련 등이 알려져 있으면 림프종, 결핵 등이 있다. 하지만 골수에서 보이는 육아종성 병변은 대부분 질병특유적(pathognomonic)으로 나타나는 것이 아니므로 이에 대한 진단은 임상적인 상황을 고려해야 할 것이다. World Association of Sarcoidosis and Other Granulomatous Disease (WASOG) 유육종증 장기 평가도구에 따르면, 본 증례의 환자는 확률 90%로 유육종증의 재발로 진단되었고, 골수의 육아종성 병변과 범혈구감소증, 점상출혈의 임상증상이 이 진단을 보조하였다.
Sarcoidosis is characterized by systemic, non-caseating epithelioid granulomas [1], which are resultant of an overactive immune system. Sarcoidosis commonly affects the lungs, with more than 90% of patients presenting pulmonary manifestations [2]. The World Association of Sarcoidosis and Other Granulomatous Disease (WASOG) created a new tool for sarcoidosis organ involvement assessment including newer diagnostic criteria for organ involvement and diagnostic techniques for a more precise clinical evaluation [3]. Bone marrow involvement is rare, accounting for <10% of sarcoidosis organ involvement, and isolated extrapulmonary sarcoidosis occurs in less than 5% of cases [4]. In the present study, we report a case of bone marrow involvement in a patient with relapsed sarcoidosis. Because there is no pathognomonic laboratory test or histological appearance for sarcoidosis, its diagnosis is commonly overlooked [1]. Further, we review the differential diagnosis of bone marrow granuloma and its clinical characteristics, which could provide new insights into diagnosing bone marrow involvement in sarcoidosis by applying the WASOG instrument. This study was approved by the Korea University Guro Hospital Institutional Review Board (IRB No. 2020GRO414).
A 50-year-old Asian woman presented with a 10-day history of insidious onset of petechia on both extremities and normal vital signs. The petechia were first noticed in the legs and, within 5 days, it appeared on the arms. The patient also reported tonsil enlargement 5 days prior to admission, which subsidized without medication. The patient had a past medical history of sarcoidosis, which was incidentally found on chest radiography as bilateral hilar lymphadenopathy and para-aortic enlargement at the age of 38 years (year 2004). Bilateral hilar adenopathy on chest radiography, especially without symptoms such as fever, night sweats, or weight loss, suggests a diagnosis of sarcoidosis, even without tissue confirmation [5]. At the time, mediastinal and hilar lymph node and arm soft tissue biopsies were performed, all of which showed chronic, non-caseating granulomatous inflammation. To rule out lymphoma, sarcoidosis, and tuberculosis lymphadenopathy, several tests were performed, including tuberculosis test (TB-PCR), serum angiotensin converting enzyme (ACE) level assessment, and bronchoscopy, all of which were normal. The patient underwent systemic steroid treatment until 2010. The size and extent of the lung lesions in 2004 were all within the normal limits. The patient remained free of any disease symptoms until 2014.
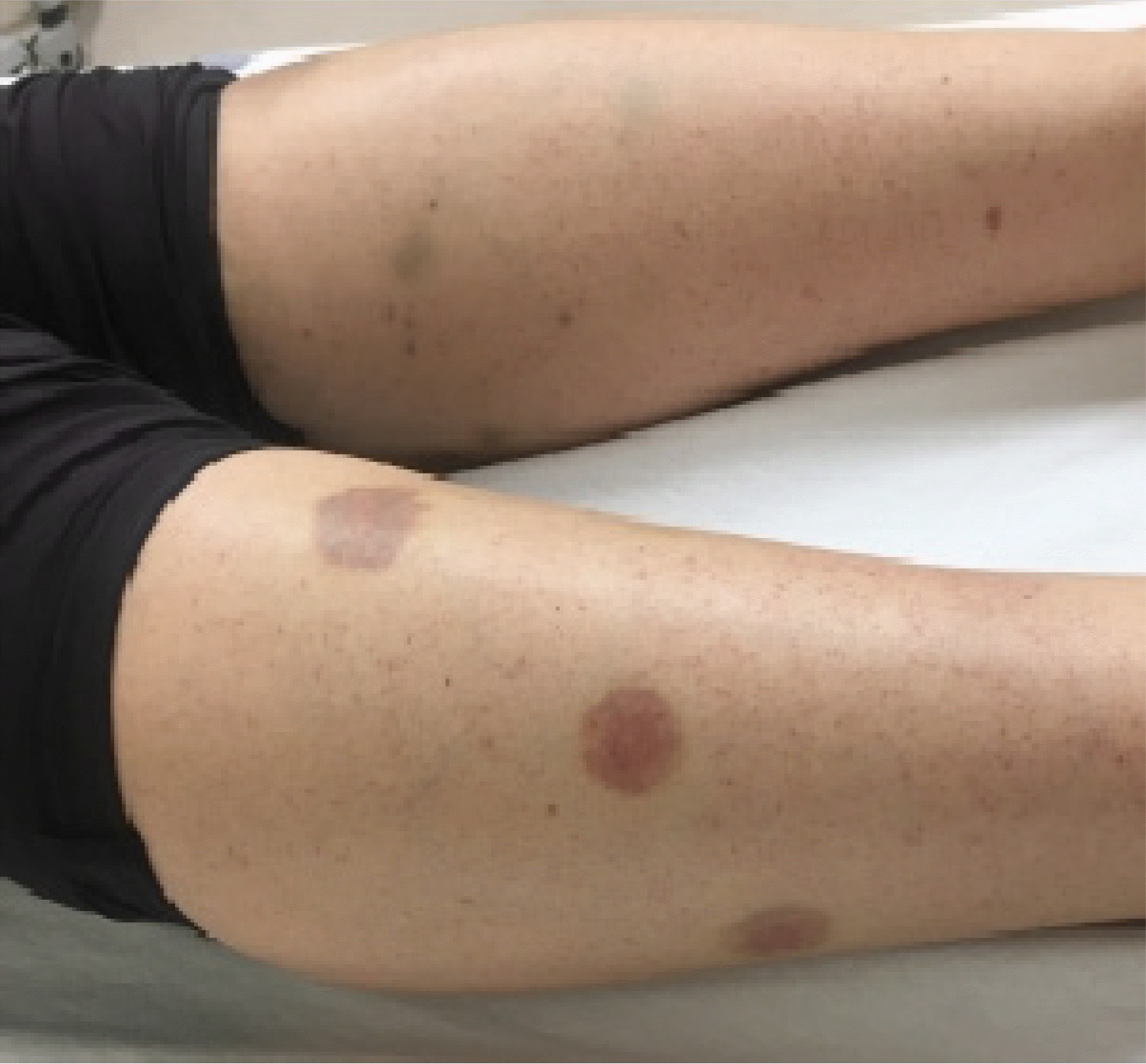
On the day of admission, multiple coin-sized violaceous to brownish papules and plaques over both extremities were observed (Fig. 1). No peripheral lymphadenopathy or organomegaly was observed. Laboratory parameters were significant for pancytopenia with severe neutropenia (absolute neutrophil count, 66/µL) and relative lymphocytosis (92%). The complete blood count was as follows: hemoglobin, 10.5 g/dL; white blood cells, 1.1×109/L; platelets, 6×109/L; normocytic normochromic anemia was present. The inflammatory marker C-reactive protein was elevated (20.89 mg/L). Moreover, serum haptoglobin was increased (266 mg/dL), reticulocyte count was decreased (0.20% of red blood cells), and plasma hemoglobin was normal, which ruled out a diagnosis of hemolytic anemia.
Further hematological studies revealed increased serum iron (227 µg/dL) and ferritin (477.10 ng/mL) levels, and decreased total iron binding capacity (243 µg/dL). ACE, Ca levels, coagulation tests, and liver function tests were within the normal limits. Other tests, including for the human immunodeficiency virus (HIV) p24 antigen and HIV-1/2 antibody, antinuclear antibody, venereal disease, and hepatitis C and B viruses, were unremarkable. The bone marrow karyotype was normal.
Chest posteroanterior assessment showed no pathologic findings other than the decreased bilateral hilar lymphadenopathy detected in 2004. Chest computed tomography (CT) showed bilateral symmetric homogeneous lymphadenopathy in both the hilum and mediastinum, all of which decreased in size and extent. Positron emission tomography (PET)-CT showed focal hypermetabolic lesions in the bilateral neck level IIa and IIb and mild hypermetabolism in the thorax, spleen, and right iliac bone area. Magnetic resonance imaging of the brain and lumbar spine did not show evidence of neurosarcoidosis, cord compression, or neural impingement.
Special stains for fungi and mycobacteria were unremarkable, ruling out other causes of non-caseating granuloma on the bone marrow biopsy. Flow cytometric analysis revealed no evidence of T- or B-cell lymphoma or acute leukemia. Sarcoidosis was by exclusion, presumed to be the diagnosis in this case.
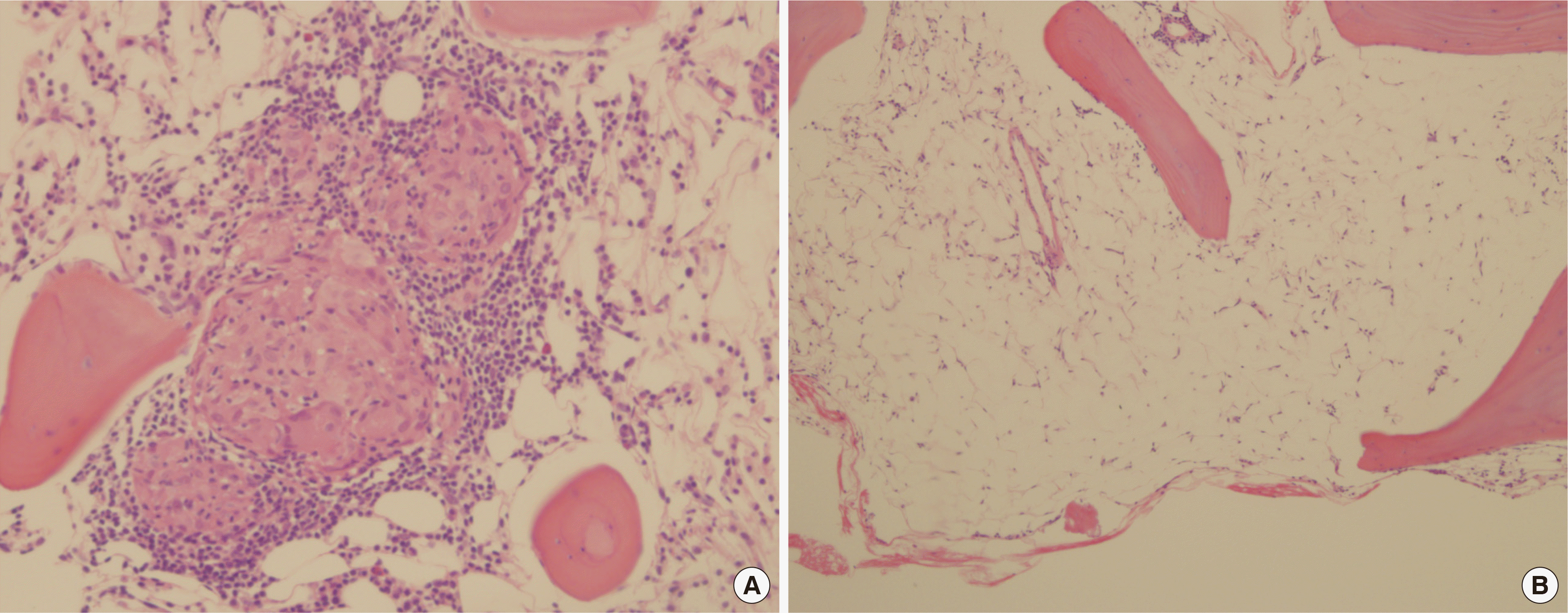
A bone marrow biopsy showed a few scattered, well-formed focal granulomas with lymphocyte aggregates (Fig. 2A), consistent with a diagnosis of sarcoidosis. Biopsy cellularity was decre-ased for age, with cellularity of less than 10% (Fig. 2B). Immunohistochemical staining for c-kit and CD34 revealed few scattered blast cells. Glycophorin-positive erythroid cells were decreased in number. The number of myeloperoxidase-positive myeloid cells was markedly decreased. Megakaryocytes were rarely observed. Reticulin stain showed no myelofibrosis (MF-0). A bone marrow aspiration smear showed an myeloid:erythroid ratio of 1.9:1. Erythroid and granulocytic precursors were markedly decreased in number and normal during maturation. Lymphocytes were relatively increased, up to 82.1% of the marrow all nucleated cells.
By applying the WASOG sarcoidosis organ assessment instrument, granulomas found in the bone marrow biopsy, normal kar-yotype, sarcoidosis history, steroid response, imaging studies, and the newly found clinical symptoms (petechia and pancytopenia) suggested a diagnosis of relapsed sarcoidosis with bone marrow involvement.
Sarcoidosis is characterized by non-caseating granulomas in multiple organs, including the lungs, lymph nodes, skin, and eyes. It is a rare systemic inflammatory disorder of unknown etiology. Sarcoidosis is exceedingly rare in Korea, with studies in Japan and Korea reporting a relatively low incidence of sarcoidosis, accounting for 0.13-1.01% per 100,000 population [6]. Bone marrow involvement in relapsed sarcoidosis is extremely rare, accounting for 0.3% of cases [7].
The diagnosis of sarcoidosis is not definitive [5]. Sarcoidosis organ involvement may not cause any significant clinical symptoms and is often underdiagnosed [8]. Judson suggested a multistep process involving the comprehensive collection of clinical information, histologic examination of tissue for the presence of granulomatous inflammation, exclusion of other known causes of granuloma formation, and documentation that granulomatous inflammation is present in at least two organs [5].
The WASOG organ assessment instrument, developed in 2014, assesses various clinical manifestations of the probability of sarcoidosis organ involvement. Two criteria are required to apply this assessment: 1) histologic evidence of granulomatous inflammation of unknown cause in an organ that is not being assessed; 2) alternative causes for the clinical manifestation being addressed other than sarcoidosis have been reasonably excluded [9]. For all organs, a biopsy showing granulomatous inflammation where alternative causes were reliably excluded was considered “highly probable,” and the likelihood of sarcoidosis was at least 90% [9]. A “highly probable” clinical manifestation of the lung was bilateral hilar adenopathy, perilymphatic nodules, and symmetrical hilar/mediastinal adenopathy in imaging studies, which our patient manifested in 2004, in addition to the histologic evidence of granulomatous inflammation. For the WASOG assessment of bone marrow involvement in sarcoidosis, a “highly probable” criteria includes PET displaying diffuse uptake [9]. Moreover, a complete blood count has been suggested as an adjunct to determine bone marrow involvement [9].
In our case, a history of sarcoidosis, skin manifestation, and pancytopenia were considered strong indicators of bone marrow involvement. Papules/plaques and subcutaneous nodules are the most common sarcoidosis-specific skin lesions [10]. Subcutaneous nodules are caused by granulomatous inflammation of adipose tissue underneath the skin and are commonly found on the extremities [10]. In addition, unexplained anemia, leukopenia, and thrombocytopenia are considered definite clinical findings in the clinical criteria for extrapulmonary sarcoidosis organ involvement [5]. High incidences of anemia, leukopenia, and extrapulmonary involvement have been reported in bone marrow involvement in sarcoidosis [12]. Anemia (hemoglobin <11 g/dL) occurs in 4-20% of patients with sarcoidosis, and leukopenia occurs in 40% of patients [13].
Leukopenia alone may be the initial presentation of sarcoidosis secondary to bone marrow infiltration or lymphocyte redistribution [7]. Unexplained cytopenia, although nonspecific, may be a solitary finding and marker; thus, clinicians should maintain a high index of suspicion [7, 12]. Granulomatous infiltration of the bone marrow is the cause of cytopenia [11]. According to the WASOG instrument, pancytopenia is considered an adjunct to determine bone marrow involvement [9].
The presence of non-caseating granuloma on biopsy is not pathognomonic of sarcoidosis because several other diseases can cause similar histopathologic changes [13]. This statement points to the complementary clinical information needed to make the diagnosis of sarcoidosis and that pathological findings of a granuloma are insufficient [5]. The estimated incidence of granulomas in bone marrow biopsies is low, accounting for 0.3-2.2% of all bone marrow biopsies. Sarcoidosis accounts for up to 21% of these cases [7]. Bone marrow granulomas have been reported in malignant neoplasms, tuberculosis, infectious mononucleosis, infectious causes such as brucellosis, typhoid fever, Q fever, tuberculosis, acquired immunodeficiency syndrome, Rickettsial disease, drug use, and autoimmune diseases [14]. The diagnosis of sarcoidosis requires that all these diseases should be excluded to a reasonable degree, which at least requires special staining and culture of the specimen for mycobacteria and fungi [5]. Malignant neoplasms have been reported as the cause of bone marrow granulomas in 20-25% of cases, most of which are malignant lymphomas of the Hodgkin’s and non-Hodgkin’s types [14]. If medical history suggests a possible alternative diagnosis, additional tests may need to be performed. In our case, we performed bone marrow cultures and stains to exclude fungal and mycobacterial infections. Lymphomas were excluded by flow cytometry.
This study has some potential limitations. Our diagnosis process may not have been thorough. Unfortunately, the most significant limitation was that regarding time and cost, we were only able to evaluate more commonly occurring diseases that can cause granulomas such as tuberculosis and lymphoma; therefore, there was a lack of information available when excluding diseases with similar histopathologic changes. Second, the patient did not agree to further follow-up visits and investigations, and the treatment response remains unknown.
In conclusion, this case demonstrates a rare presentation of relapsed sarcoidosis with bone marrow involvement. Clinical presentations of sarcoidosis are diverse and may mimic several other diseases, posing diagnostic challenges for clinicians [10]. The diagnosis of sarcoidosis relies on the following: (1) the presence of non-caseating granulomas on histopathologic examination, (2) compatible clinical presentation, and (3) exclusion of other causes of granulomatous inflammation [10].
According to the WASOG sarcoidosis organ assessment instrument, our patient was concluded to be “highly probable,” likelihood of sarcoidosis being at least 90%, with bone marrow biopsy showing granulomatous inflammation. Excluding other causes of granuloma and systemic symptoms, all clinical findings (such as petechia and pancytopenia) supported the diagnosis of sarcoidosis.
REFERENCES
1. Akinsanya L, Hussain I, Awoniyi D, Usman K. 2008; Leucopenia as presentation of sarcoidosis. Int J Health Sci (Qassim). 2:109–12.
2. Al-Kofahi K, Korsten P, Ascoli C, Virupannavar S, Mirsaeidi M, Chang I, et al. 2016; Management of extrapulmonary sarcoidosis: challenges and solutions. Ther Clin Risk Manag. 12:1623–34. DOI: 10.2147/TCRM.S74476. PMID: 27853374. PMCID: PMC5106225.

3. Zhou Y, Lower EE, Li H, Farhey Y, Baughman RP. 2017; Clinical characteristics of patients with bone sarcoidosis. Semin Arthritis Rheum. 47:143–8. DOI: 10.1016/j.semarthrit.2017.02.004. PMID: 28274482.

4. Bickett AN, Lower EE, Baughman RP. 2018; Sarcoidosis diagnostic score: a systematic evaluation to enhance the diagnosis of sarcoidosis. Chest. 154:1052–60. DOI: 10.1016/j.chest.2018.05.003. PMID: 29778660. PMCID: PMC6859250.
5. Judson MA. 2008; The diagnosis of sarcoidosis. Clin Chest Med. 29:415–27. DOI: 10.1016/j.ccm.2008.03.009. PMID: 18539235.

6. Park JE, Kim YS, Kang MJ, Kim CJ, Han CH, Lee SM, et al. 2018; Prevalence, incidence, and mortality of sarcoidosis in Korea, 2003-2015: A nationwide population-based study. Respir Med. 144S:S28–34. DOI: 10.1016/j.rmed.2018.03.028. PMID: 29631888.

7. Peña-Garcia JI, Shaikh S, Barakoti B, Papageorgiou C, Lacasse A. 2019; bone marrow involvement in sarcoidosis: an elusive extrapulmonary manifestation. J Community Hosp Intern Med Perspect. 9:150–4. DOI: 10.1080/20009666.2019.1575688. PMID: 31061693. PMCID: PMC6487444.

8. Judson MA. 2016; The three tiers of screening for sarcoidosis organ involvement. Respir Med. 113:42–9. DOI: 10.1016/j.rmed.2016.02.011. PMID: 27021579.

9. Judson MA, Costabel U, Drent M, Wells A, Maier L, Koth L, et al. 2014; The WASOG sarcoidosis organ assessment instrument: an update of a previous clinical tool. Sarcoidosis Vasc Diffuse Lung Dis. 31:19–27.
10. Ungprasert P, Ryu JH, Matteson EL. 2019; Clinical manifestations, diagnosis, and treatment of sarcoidosis. Mayo Clin Proc Innov Qual Outcomes. 3:358–75. DOI: 10.1016/j.mayocpiqo.2019.04.006. PMID: 31485575. PMCID: PMC6713839.

11. Yachoui R, Parker BJ, Nguyen TT. 2015; Bone and bone marrow involvement in sarcoidosis. Rheumatol Int. 35:1917–24. DOI: 10.1007/s00296-015-3341-y. PMID: 26248533.

12. Yanardağ H, Pamuk GE, Karayel T, Demirci S. 2002; bone marrow involvement in sarcoidosis: an analysis of 50 bone marrow samples. Haematologia (Budap). 32:419–25.
13. Statement on sarcoidosis. 1999; Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 160:736–55.
14. Eid A, Carion W, Nystrom JS. 1996; Differential diagnoses of bone marrow granuloma. West J Med. 164:510–5.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download