INTRODUCTION
Urinary tract infection (UTI) is a common infectious disease of the urogenital system [
1]. UTIs are diagnosed by studying a urine culture and noting its clinical manifestations. The urine culture study has been known as the gold standard for diagnosis of UTIs, despite it being time-consuming and labor intensive. However, in patients with poor immunity, a rapid diagnostic evaluation may be required since UTIs may cause serious complications [
2,
3].
The urinalysis consists of a chemical examination using a urine strip and microscopic examination of the urine sediment, usually through automated analyzers [
4]. However, manual microscopic examination is still considered the reference method for urine sediment examination [
5]. Therefore, manual microscopic examination should be used when the results of the urine sediment analyzer are discordant with the results of the urine strip analyzer - such as RBC vs. occult blood, WBC vs. leukocyte esterase, cast vs. protein, or bacteria vs nitrite.
However, the methods of manual microscopic examination are not standardized [
6]. In addition, there is a wide inter-observer variability among laboratory technologists [
7]. According to several studies comparing automated analyzer and manual microscopic examinations, relatively good correlation was reported for cell counts of red blood cells, white blood cells, and epithelial cells but not for counts of bacteria [
8-
10]. It is important to standardize the method for bacterial counting since it can provide a clue for the diagnosis of UTI. Generally, clinical laboratories report semiquantitative methods for bacteria (negative, a few/rare, moderate, many), but there is no standardized criterion for counting them.
Cobas u 701 (Roche Diagnostics International, Rotkreuz, Switzerland) is based on auto-captured images and has recently been introduced into clinical laboratories. The detection rate of bacteria in Cobas u 701 is controlled by the analytical sensitivity of the instrument, as well as those of other cellular components [
11]. Generally, analytical sensitivity of bacteria was evaluated as part of the process for diagnostic performance at the onset of automated urine sediment analysis. The analytical sensitivity of bacteria of Cobas u 701 is primarily set at 18.18/field, but it may be increased to 30 or 50 depending on the performance evaluation results and the laboratory operating policy. Therefore, the analytical sensitivity of bacteria needs to be included as an important consideration in reporting the results of urinary bacteria.
In this study, we introduce several ways to accurately report bacteria in urine by manual microscopic examination in a clinical laboratory; the establishment of the criterion, adjustment of the analytical sensitivity of the instrument, and education about urine microscopic examination. We then evaluated whether the agreement rate between technologists increased following application of the criterion.
Go to :

MATERIALS AND METHODS
This study was carried out sequentially, beginning with establishment of the criterion for semi quantitation of bacteria in manual microscopic examination of urine, followed by analysis of the results of applying the criterion to clinical specimens. This study was approved by the Institutional Review Board of Kyung Hee University Hospital at Gangdong.
1. Selection of specimens and study design
These studies were performed on specimens with WBC 0-1/a few bacteria in the Cobas u 701 automated urine sediment analyzer. Selection of specimens and study design are shown in
Fig. 1.
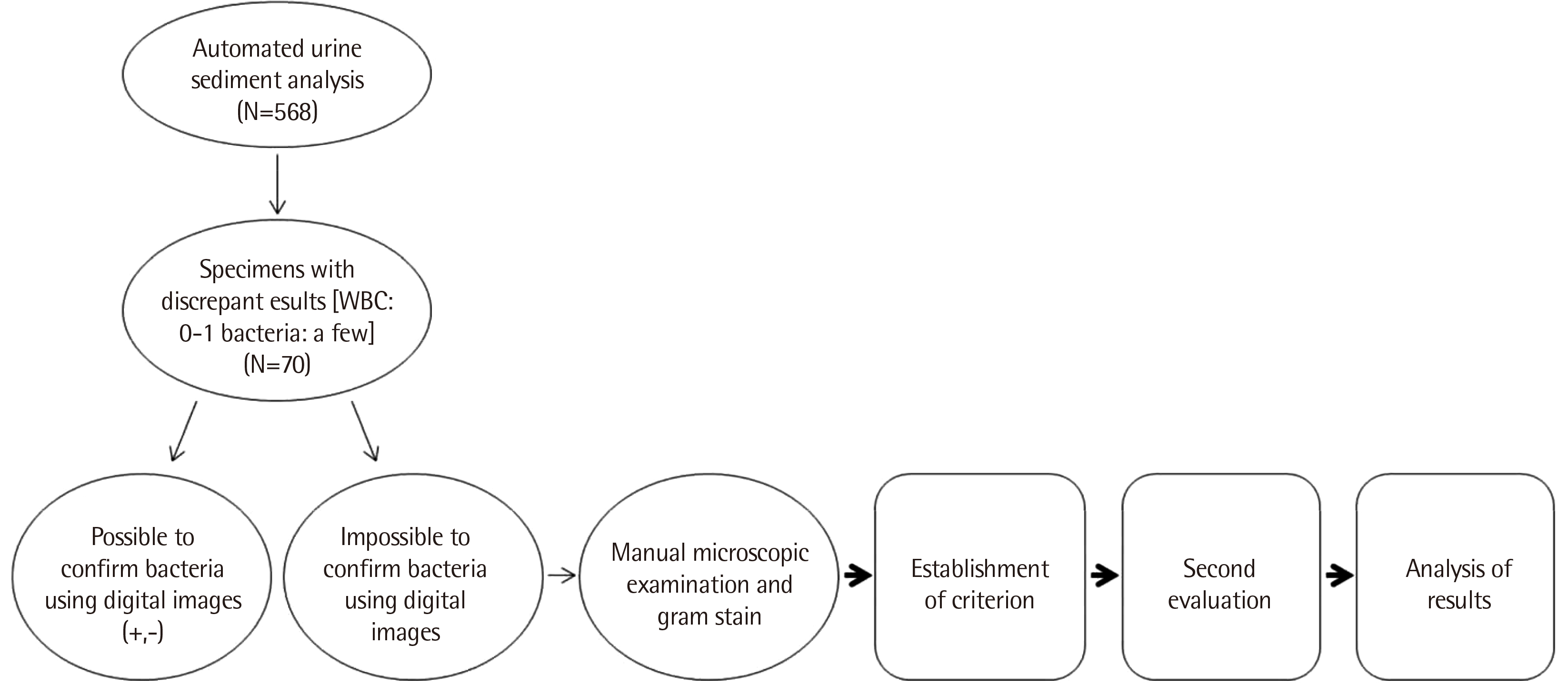 | Fig. 1
An outline of the study.
Abbreviation: WBC, white blood cell.

|
1) Analysis for establishing the criterion for semi quantitation of bacteria
Among the 568 urine specimens analyzed by the Cobas u 701 in three days, 70 (12.3%) reported results of WBC 0-1/a few bacteria. Twenty-seven specimens (38.6%) could be interpreted with digital images stored in Cobas u 701. The technologist in charge checked the digital images and reported no bacteria as ‘negative’ and definite rod-shaped bacteria as ‘positive’ (
Fig. 2). The remaining 43 specimens (61.4%) could not be interpreted as cocci or contained artifacts such as air bubbles or dust on digital images. Further, five well-trained laboratory technologists examined urine slides using microscopy and separately recorded the results of semi quantitation of bacteria. Gram staining was also performed on these specimens.
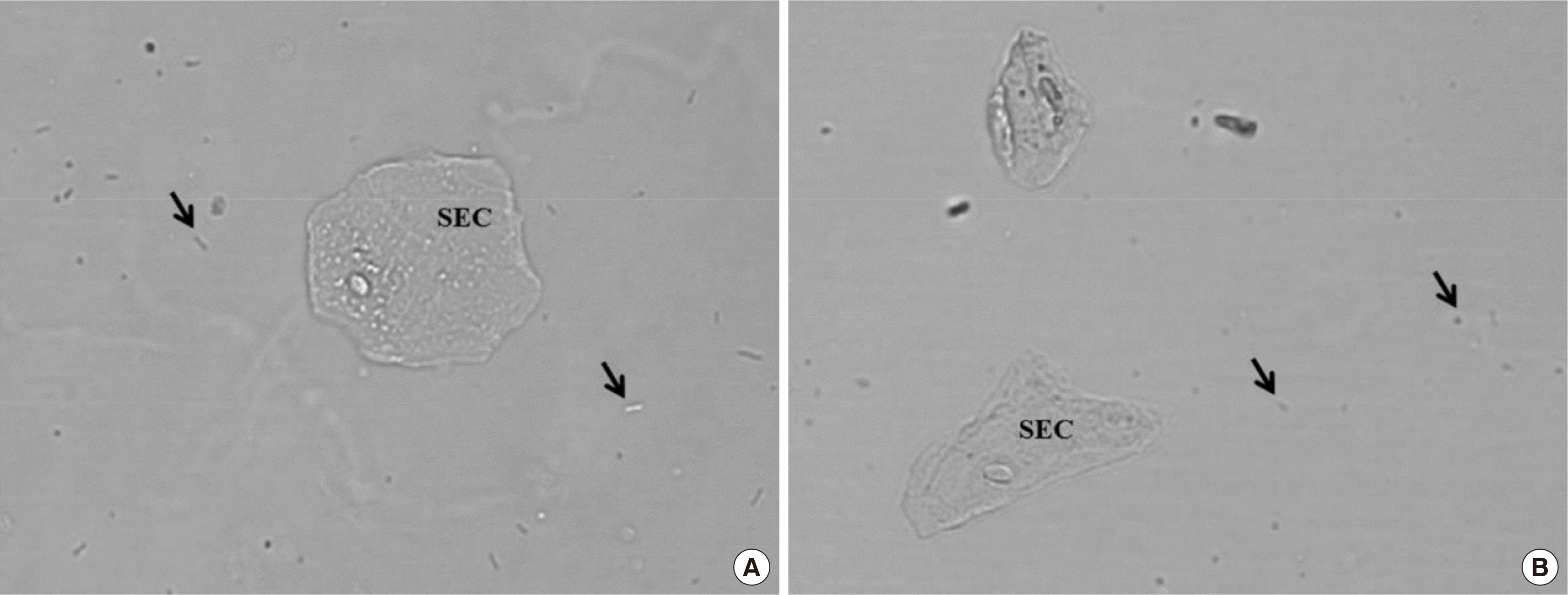 | Fig. 2
Digital images from Cobas u 701. (A) Arrows indicate rod bacteria. (B) Arrows indicated cocci-like features, but were confirmed as artifacts by manual microscopic examination.
Abbreviation: SEC, squamous epithelial cell.

|
2) Establishment of the criterion
We assessed the five technologists’ individual criteria for semi quantitation of bacteria, and the results of the manual microscopic examinations were compared with those of the Gram stains. We established the criterion for semi quantitation of bacteria during manual microscopic examinations, considering these results.
3) Evaluation of usefulness of established criterion
Among the 1,966 urine specimens analyzed by Cobas u 701 in two weeks, 215 (10.9%) reported results were of WBC 0-1/a few bacteria. A total of 71 specimens were subjected to manual microscopic examination by the same five technologists, after excluding 143 specimens that could be interpreted as having negative or positive results by the digital images of the Cobas u 701. On these 71 specimens, Gram staining and a urine culture were also conducted. We evaluated the agreement rate between technologists and analyzed the sensitivity and specificity of manual microscopic examination based on the results of urine culture and Gram staining.
2. Urine sediment analysis using Cobas u 701
A sample of 200 μL of well-mixed un-centrifuged urine was transferred to a cuvette and centrifuged in the analyzer. Fifteen high-quality digital images were captured and interpreted by Auto Image Evaluation Module software (Roche Diagnostics International). For bacteria, Cobas u 701 reported semiquantitative results as negative, a few, moderate, or many.
3. Manual microscopic examination of urine
Ten milliliters of well-mixed urine was centrifuged (1,500 rpm, 5 minutes). Following this, the supernatant was decanted, and the remaining 150 μL was re-suspended. One drop was placed on the slides and covered with an 18×18 mm cover slip. The smear was examined using microscopy under high-power field (HPF, ×400). Before establishment of the new criterion, the results were reported according to the individual criterion of the five technologists. However, for evaluation of the established criterion, the mean value of the 5 HPFs was calculated and reported according to the newly established criterion (negative: <20, a few: 20–30, moderate: 31–49, many: ≥50 per HPF). The results of ‘agreement’ were defined as four or more of the five technologists reporting the same results.
4. Gram staining
One drop (about 50 μL) of urine was placed on a slide and allowed to air dry. The smear was stained with Gram stain and examined for the presence of bacteria under an oil immersion microscope (×1,000 magnification). A positive result was defined as the presence of ≥1 bacteria/oil immersion field [
12,
13].
5. Urine culture
A urine culture was performed by inoculating 1 μL of urine onto a 5% sheep blood agar plate and MacConkey agar (Bi-plate [BAP/MAC], Asan Pharmaceutical Co., Yongin, Korea) and streaking the entire plate surface. The agar plates were incubated aerobically at 35°C for 24 hours. Positive results were defined as the presence of colony forming units ≥105/mL. No growth or less than 105/mL was considered negative. Three or more isolates without a dominant pathogen was regarded as contamination.
Go to :

DISCUSSION
Urine sediment analysis is one of the two axes of urinalysis and is performed with the urine strip test. Urine sediment analysis is traditionally based on microscopic examination. Findings about erythrocytes, leukocytes, various epithelial cells, casts, crystals, bacteria, yeasts and other elements are reported. However, introduction of the automated urine sediment analyzer in 1982 allowed faster and more precise analysis [
15]. Most automated urine sediment analyzers in clinical laboratories adapted a flow cytometry or image-based method as the principle [
8]. Cobas u 701 detects urine sediments using 15 digital images captured by a digital camera. They report quantitative results for RBC or WBC and semiquantitative results for bacteria, epithelial cells, and hyaline casts. For pathological casts, crystals, yeasts, mucus, and sperm, qualitative data are provided. In cases where it is necessary to confirm the results, technologists can examine the stored digital images. However, manual microscopic examination should be performed when it is difficult to interpret results using the images. It is not easy to interpret the existence of bacteria by only digital images [
16-
18]. Correct detection of cocci is more difficult than that of rod forms, digitally [
11]. Therefore, manual microscopic examination is an essential procedure in a clinical laboratory, and there is a need for standardization of the criterion for semi quantitation to obtain an accurate diagnosis of UTI.
However, a standardized criterion has not been established. Several studies reported sensitivity and specificity according to various lower limits for positive results of bacteria. When the criterion of positivity was set to ≥1/HPF, the sensitivity was greater than 90% and specificity was 50-80% [
19,
20]. In the same article, when the criterion was set to ≥100/HPF, the sensitivity was 60-80% and the specificity was almost 100%. In a review article, the authors suggested four categories: negative, <1/HPF, ≥1 and ≤50/HPF, and >50/HPF [
21]. In another literature, the suggested lower limit of positivity was 20/HPF [
14].
We established the criterion by considering the technologists’ individual criteria and by analysis of comparative data of manual microscopic examination and Gram staining. There was weak agreement (58.2%) between the technologists because no clear-cut criterion for bacterial counting existed. Although the sensitivity was not high, the specificity was very low at 32.5%. This result implied that there was a high rate of false positivity of manual microscopic examination. At that time, the clinicians at our hospital complained that there was a tendency of ‘a few’ bacteria in urine sediment analysis even in patients with very low necessity for retest or further examination. We assessed each criterion of the five technologists and raised the lower limit of the ‘a few’ result to ≥20/HPF.
When the newly established criterion was applied, the specificity increased to 87.7% with the same sensitivity (66.7%). The sensitivity or specificity of Cobas u 701 based on urine culture was not analyzed in this study. However, in a comparison study of diagnostic performance of bacteria for Cobas u 701 and urine culture, the sensitivity and specificity were 81.5% and 73.8%, respectively (with analytical sensitivity of 30/field) [
11]. Based on the two studies (this study and reference [
11]), there was no statistical difference in sensitivity between Cobas u 701 and manual microscopic examination, but specificity was significantly higher in manual microscopic examination (
P<0.05). In addition, following the new criterion, the PPV of manual microscopic examination for Gram stain was improved significantly from 18% to 70%. Consequently, patients with positive results of urinary bacteria by manual microscopic examination were more likely to have UTI clinically when applying the new criterion.
Further studies with more specimens with positive results of urine culture are needed. With the establishment of the new criterion, it is also necessary to ensure that technologists can distinguish between cocci and other artifacts in urine microscopic examination and to educate them to interpret the results correctly. Further, since the detection rate varies depending on the analytical sensitivity of the analyzer, adjustment of analytical sensitivity should be considered when the false positive rate of the analyzer is high [
11]. Thus, we adjusted the analytical sensitivity of the Cobas u 701 from 18.18/field to 30/field.
In this study, we targeted specimens with initial results of WBC 0-1/a few bacteria from the automated urine sediment analyzer. It can be difficult to determine whether that result is to be ignored as contamination or to be retested, especially for women. Therefore, in these cases, confirmation of the actual existence of bacteria is needed. During the study period, these cases comprised about 10% of the sample population. Among them, 59.6% ([27+143]/[70+215]) of the specimens could be interpreted on stored digital images (positive results: 46.2%, data not shown). A total of about 40% of specimens were targeted for manual microscopic examination.
The limitation of this study is that there was a small number of specimens with positive results from urine culture. Therefore, even though the lower limit before establishment of the new criterion was low (4-10/HPF), the sensitivity was lowered to 66%. There is a need to analyze more specimens with positive results of urine culture. In addition, the amount of urine observed in urine sediment analyzer, manual microscopy, and Gram stain was different. Further study is needed because the amount of urine used in the test may affect the sensitivity or specificity of the test.
This study suggests several important points. Through this study, it can be seen that each technologist has a different standard for semi quantitation of urine bacteria in manual microscopic examination. Moreover, when the new criterion was established by applying the experimental results with appropriate education, more clinically useful information could be reported. Additionally, the process is described in detail so that it can be practically applied to other clinical laboratories.
In clinical laboratories, it will be necessary to ensure that all technologists are applying the same criterion. In addition, it is necessary to assess the analytical sensitivity of the automated urine sediment analyzer and the need for education about the correct interpretation of urine microscopic examination.
Go to :





 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download