Abstract
Long-chain (LC) n-3 polyunsaturated fatty acids (n-3 PUFAs), in particular docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), are nutrients involved in many metabolic and physiological processes, and are referred to as n-3 LCPUFA. They have been extensively studied for their effects in human nutrition and health. This paper provides an overview on metabolism, sources, dietary intake, and status of n-3 LCPUFA. A summary of the dietary recommendations for n-3 LCPUFAs for different age groups as well as specific physiological conditions is provided. Evidence for n-3 LCPUFA in cardiovascular diseases, including new studies, is reviewed. Expert recommendations generally support a beneficial effect of n-3 LCPUFA on cardiovascular health and recommend a daily intake of 500 mg as DHA and EPA, or 1–2 servings of fish per week. The role of n-3 LCPUFA on brain health, in particular neurodegenerative disorders and depression, is reviewed. The evidence for beneficial effects of n-3 LCPUFA on neurodegenerative disorders is non-conclusive despite mechanistic support and observational data. Hence, no definite n-3 LCPUFA expert recommendations are made. Data for the beneficial effect of n-3 LCPUFA on depression are generally compelling. Expert recommendations have been established: 200–300 mg/day for depression; up to 1–2 g/day for major depressive disorder. Recent studies support a beneficial role of n-3 LCPUFAs in reducing the risk for premature birth, with a daily intake of 600–800 mg of DHA during pregnancy. Finally, international experts recently reviewed the scientific evidence on DHA and arachidonic acid (ARA) in infant nutrition and concluded that the totality of data support that infant and follow-on formulas should provide both DHA and ARA at levels similar to those in breast milk. In conclusion, the available scientific data support that dietary recommendations for n-3 LCPUFA should be established for the general population and for subjects with specific physiological conditions.
Go to : 
Scientific interest in the health benefits of n-3 long-chain polyunsaturated fatty acids (n-3 LCPUFAs), often also referred to as omega-3 LCPUFA, has resulted over recent decades in an extensive set of scientific data that is focused on their functional role in cardiovascular disease (CVD), brain functionality and performance, as well as other functional roles relevant for human health (e.g., immune response, allergy) or related to particular physiological states (e.g., pregnancy, prematurity, infancy).
With noncommunicable diseases (NCDs) on the rise, estimated globally to be the cause of death for 38 million people each year, focus on the role of the diet has increased, given it is considered a main contributor to managing the incidence of NCD [1]. Therefore, health authorities such as the World Health Organization focus on strategies to reduce the incidence NCDs, which include dietary guidance as a key factor in the management of NCDs at both global and national levels [2]. Dietary n-3 LCPUFA, eicosapentaenoic acid (EPA, 20:5n-3), and docosahexaenoic acid (DHA, 22:6n-3), have been associated with a decreased risk of chronic diseases, in particular CVD [3] and cognitive decline [4]. Another area of considerable research has focused on the functional role of n-3 LCPUFA during the first 1,000 days of life, a period of rapid growth and development [567]. Despite overall positive data on a beneficial role of n-3 LCPUFA in human health, there is no consistent, global recommendation for dietary intake of n-3 LCPUFA to manage nutritional needs and reduce NCD risk.
The present paper reviews the available evidence, particularly recent scientific evidence, in support of n-3 LCPUFA intake and the intake-related recommendations developed by experts. To this aim this paper provides a brief overview of the metabolic and functional role of n-3 LCPUFA, the associated nutritional recommendations, dietary intake and status, and it reviews in more detail recent data related to the role of n-3 LCPUFA in CVD, brain functionality, specific dietary needs during the first 1,000 days of life, and other selected conditions that are of public health interest.
Go to : 
Omega-3 polyunsaturated fatty acids (n-3 PUFAs) are PUFAs with one of its double bonds located 3 carbon atoms from the methyl end. The quantitatively most important n-3 PUFA in the diet are: 18:3n-3 (alpha-linolenic acid, ALA), 20:5n-3 (EPA), 22:5n-3 (docosapentaenoic acid, DPA), and 22:6n-3 (DHA). The n-3 LCPUFA are involved in many physiological and metabolic processes and hence play a key role in human metabolism. They also are important structural components of cell membranes and contribute to various membrane functions such as fluidity, permeability, activity of membrane-bound enzymes and receptors, and signal transduction.
ALA is essential in human nutrition as a precursor for n-3 LCPUFA. The EPA, DPA, and to a lesser degree DHA are synthesized from ALA through the sequential action of various desaturases and elongases in animal tissues, but not in plants (Fig. 1). Estimates for the conversion of ALA into EPA range from 8% to 12%, while its conversion into DHA may be less than 1% [8]. Due to this low conversion and the fact that ALA, and EPA and DHA may have different biological functions, many authorities have made separate recommendations for ALA on the one hand, and for EPA and DHA on the other hand. EPA is the precursor for series 3 prostanoids and series 5 leukotrienes [9], while DHA is a component of membrane structural lipids, especially phospholipids in nervous tissue and retina. The developing brain accumulates large amounts of DHA both pre- and postnatally, particularly during the first 2 years of life, with DHA predominantly acquired from the mother via placental transfer and breast milk. Moreover, the capacity of the brain to synthesize DHA increases with gestational age [1011].
Both n-3 and n-6 LCPUFA are synthesized in the body through conversion of their essential precursors ALA and linoleic acid (LA) by the same enzymes. The conversion of ALA into EPA and DHA is decreased when the amount of LA in the diet increases (and vice versa). For this reason, some dietary recommendations also include guidelines for a desired n-3/n-6 ratio in the diet. While the proportion of dietary ALA converted into n-3 LCPUFA is not influenced by the dietary n-3/n-6 ratio, the amounts of n-3 LCPUFA formed depends on the amount of ALA consumed [8].
The ability to convert ALA into n-3 LCPUFA and the levels of n-3 LCPUFA in plasma phospholipids and red blood cells are, moreover, individually related to polymorphisms in the human Δ-5 and Δ-6 desaturase genes fatty acid desaturase 1 and 2 (FADS1 and FADS2, respectively) [12]. Polymorphisms in the FADS genes determine the efficiency of endogenous PUFA processing, and, therefore, nutritional and functional responses are likely to vary depending on the FADS genotype present.
Fish, in particular fatty fish, is a rich source of n-3 LCPUFA (EPA and DHA). Other natural sources are cultivated marine algae (single cell oils) and human milk, an important source for infants. EPA and DHA may also be provided by foods and supplements that have been enriched with n-3 LCPUFA. ALA, the precursor of n-3 LCPUFA, is found in some vegetable foods, for example linseeds, rapeseed oil, and walnuts.
The dietary intake of n-3 LCPUFA is largely dependent on dietary habits and hence has been reported to show considerable geographical and population differences [13]. Differences in dietary intake result in differences in n-3 LCPUFA status. Global dietary intakes of n-3 LCPUFA has been examined, and it has been estimated that less than 20% of the world's human population consume ≥ 250 mg/day of seafood-origin n-3 LCPUFA. However, the reliability of the EPA and DHA intake estimates is limited by various factors, including the availability of accurate and timely food composition data in nutrient databases that may differ across countries [1314], as well as by the challenge of reporting errors in the collection of dietary data [1516].
Markers for n-3 LCPUFA status, such as the fatty acid composition of blood, do not have the above noted limitations, and blood EPA + DHA levels reflect other metabolic factors and behavioral choices that can influence EPA + DHA status [171819202122]. Blood levels of n-3 LCPUFA, particularly EPA and DHA, have been linked to a reduced risk of primary cardiac arrest [22], sudden cardiac death [23], and all cause dementia [24]. Stark and colleagues [25] conducted a systematic review of the available blood EPA + DHA levels to create a visualization of global EPA + DHA status that could identify countries and regions potentially at an increased risk of chronic disease due, at least in part, to their n-3 LCPUFA status. That review included 298 studies reporting n-3 LCPUFA levels in various blood fractions of healthy adults published in 1980 and later. The fatty acid data from each blood fraction were converted to relative weight percentages (wt.%) and then assigned to one of 4 discrete ranges (high, moderate, low, very low) corresponding to wt.% EPA + DHA values in erythrocyte equivalents. Data revealed that geographical regions with high EPA + DHA blood levels (> 8%) included countries adjacent to the Sea of Japan, Scandinavia, and areas with indigenous populations or populations not fully adapted to Westernized food habits. Very low blood levels (≤ 4%) were observed in North, Central, and South America, Europe, the Middle East, Southeast Asia, and Africa. The authors concluded that, globally, there is considerable variability in EPA + DHA blood levels, and that the very low to low range of blood EPA + DHA levels for most regions of the world may increase global risk for chronic disease.
Nutritional recommendations for n-3 LCPUFA consumption for different age groups, as well as for selected specific disease related conditions such as CVD, have been established by several expert authorities.
In 2010, recommendations of the Joint Food and Agriculture Organization of the United Nations/World Health Organization Expert Consultation on Fats and Fatty Acids in Human Nutrition, including recommendations for n-3 LCPUFA, were published [26]. Those recommendations reflected the clear recognition of an increasing global burden of nutrition-related chronic disease. The expert consultation concluded that a daily ALA intake of 0.5–0.6% of energy is required to cover nutritional needs. The total n-3 fatty acid intake can range between 0.5% and 2% of energy, with the minimum dietary requirement of ALA at > 0.5% of energy for adults considered to prevent deficiency-related symptoms. The higher value of 2% of energy for ALA plus n-3 LCPUFA (EPA + DHA) (i.e., 0.25–2.0 g) can be considered indicative of a healthy diet. The expert consultation also concluded that there is evidence that n-3 LCPUFA may contribute to the prevention of coronary heart disease and possibly other degenerative diseases related to aging. For adult males and non-pregnant/non-lactating adult females, 0.25 g/day of EPA plus DHA is recommended, given there is insufficient evidence to establish a specific minimum intake for either EPA or DHA alone. For adult pregnant and lactating females, the minimum intake for optimal adult health and fetal and infant development is 0.3 g/day EPA + DHA, of which at least 0.2 g/day should be DHA. The upper safe intake level for EPA + DHA was set at 2 g/day based on experimental evidence indicating that a high intake of n-3 LCPUFA may increase lipid peroxidation and reduce cytokine production. However, it was also acknowledged that a high n-3 LCPUFA consumption of up to 3 g/day could reduce other cardiovascular risk factors and did not have any adverse effects in short- and intermediate term randomized trials. Moreover, some individuals in populations with high seafood consumption may consume even higher values with no apparent evidence of harm.
The European Food Safety Authority (EFSA) established recommendations for total fat and fatty acids, including n-3 LCPUFAs, in 2010 [27]. The EFSA set an adequate intake level for ALA at 0.5% of energy and did not identify a upper safe level. An adequate intake for EPA + DHA was established for adults at 250 mg/day. Similarly, an adequate intake of 100 mg/day was set for DHA for infants (> 6 months) and young children < 24 months. For pregnancy and lactation, a daily intake of 100–200 mg of DHA, in addition to the adult adequate intake, was established to meet the increased demand for n-3 LCPUFA. The EFSA also investigated the tolerable upper intake level (UL) and concluded that the available data were insufficient to establish an UL for n-3 LCPUFA (individually or combined) for any population group [28]. At the observed intake levels, consumption of n-3 LCPUFA has not been associated with adverse effects in healthy children or adults. Long-term supplemental intakes of EPA and DHA combined of up to about 5 g/day do not appear to increase the risk of spontaneous bleeding or bleeding complications, nor do they affect glucose homeostasis, immune function, or lipid peroxidation, provided the oxidative stability of the n-3 LCPUFAs is guaranteed. Supplemental intakes of EPA and DHA combined at doses of 2–6 g/day, or of DHA at doses of 2–4 g/day, induce increases in low-density lipoprotein (LDL)-cholesterol concentrations of about 3%, which may not have an adverse effect on CVD risk, whereas EPA at doses up to 4 g/day has no reported significant effect on LDL-cholesterol. Supplemental intakes of EPA and DHA combined at doses up to 5 g/day, and supplemental intakes of EPA alone up to 1.8 g/day, do not raise safety concerns for adults. Dietary recommendations for EPA and DHA consumption, based on cardiovascular risk considerations for European adults, are between 250 and 500 mg/day. Supplemental intakes of DHA alone of up to about 1 g/day do not raise safety concerns for the general population. No data are available for DPA when consumed alone. In the majority of the human studies reviewed, fish oils, also containing DPA in generally unknown (but relatively low) amounts, were the source of both EPA and DHA.
The Institute of Medicine (IOM) of the National Academies established adequate intake levels for ALA only as this was considered the only n-3 fatty acid that is nutritionally essential [29]. However, for infants (0–1 year) the adequate intake applies to the total of all n-3 fatty acids. The IOM did not establish specific intake recommendations for EPA, DHA, or other n-3 LCPUFA, but it did recommend that approximately 10% of the Acceptable Macronutrient Distribution Range for ALA can be consumed as EPA and/or DHA. This recommendation is similar to the current mean intakes of EPA and DHA in the United States (US) (approximately 100 mg/day), an intake that is much lower than that being recommended by many groups worldwide. Global recommendations for n-3 LCPUFA underscore the pressing need to establish dietary reference intakes (DRIs) for DHA and EPA because DRIs are recognized as the “official” standard by which federal agencies issue dietary guidance or policy directives for the health and well-being of individuals in the US and Canada. Because of the many health benefits of DHA and EPA intake, it is important and timely that National Academies establish DRIs for the individual long-chain (20 carbons or more) n-3 fatty acids. The IOM recognizes that previous research indicates that the consumption of fish and other types of seafood as part of a balanced diet can promote “heart health”. Fish oil and other n-3 LCPUFA supplements improve blood lipids and appear to reduce the risk of cardiac death. However, their effects on other cardiovascular endpoints are unclear and might vary based on dietary n-3 intake levels and the use of cardioprotective medications. Consequently, the US Food and Drug Administration (FDA) has approved a qualified health claim for conventional foods and dietary supplements that contain EPA and DHA [30]. It states, “Supportive but not conclusive research shows that consumption of EPA and DHA omega-3 fatty acids may reduce the risk of coronary heart disease.” The FDA also specifies that dietary supplement labels should not recommend a daily intake of EPA and DHA higher than 2 g [30].
The IOM did not establish an UL for any n-3 LCPUFA, although it noted that high doses of DHA and/or EPA (900 mg/day of EPA plus 600 mg/day DHA or more for several weeks) might reduce immune function due to the suppression of inflammatory responses. Doses of 2–5 g/day EPA and/or DHA might also increase bleeding time by reducing platelet aggregation [26]. Based on the totality of evidence, the FDA recommends not exceeding 3 g/day EPA and DHA combined, with an intake of up to 2 g/day from dietary supplements [31]. It should be noted that doses used in some clinical trials exceed these levels. The 2015–2020 Dietary Guidelines for Americans and the Scientific Report of the 2015 US Dietary Guidelines Advisory Committee have recommended at least 2 servings of fish per week, providing an average of 250 mg EPA plus DHA per day, in place of other animal sources of protein [3233]. The 2015–2020 US Dietary Guidelines for Americans provide information about how to incorporate seafood into the healthy US-style eating pattern and the healthy Mediterranean-style eating pattern.
In 2017, the National Health and Medical Research Council (NHMRC) updated its earlier report (2006) on the Nutrient Reference Values for Australia and New Zealand that established recommendations for n-3 LCPUFA intake [34]. The NHMRC stated that, based on the concept of essentiality and given the lack of dose-response data, adequate intakes could be established for LA (n-6 in infants), ALA, and the combined n-3 LCPUFA, DHA + EPA + DPA, by using median population intakes in Australia. The combined EPA+DHA+DPA adequate intakes per day were set at 40 mg (1–3 years), 50 mg (4–8 years), 70 mg (9–13 years/boys), 85 mg (9–13 years/girls), 125 mg (14–18 years/boys), 85 mg (14–18 years/girls), 160 mg (men), and 90 mg (women). During pregnancy and lactation, NHMRC recognized increased needs for n-3 LCPUFA intake and estimated that 115 mg and 145 mg per day, respectively, would cover those needs.
The NHMRC also included recommendations for n-3 LCPUFA intake that could reduce the risk of chronic disease. They concluded that there is increasing acceptance of evidence that, in populations with only modest intakes of EPA and DHA, increased dietary consumption could further improve health status. Given this evidence, and the modest intakes currently consumed in Australia and New Zealand, the NHMRC considered it prudent to encourage increased consumption of n-3 LCPUFA (DHA, EPA, and DPA). Dietary intakes at the 90th centile of the population were considered adequate to provide potential benefit while also being a safe level as currently consumed by many Australians and New Zealanders. Rounding up to the nearest 10 mg, this established a level of 610 mg/day for men and 430 mg/day for women. For men, the 90th centile is close to the upper quintile from the Multiple Risk Factor Intervention Trial study, which was associated with significantly less CVDs [35], whereas for women, the 90th centile of intake is close to the level shown in the Nurses Health Study to produce a benefit [36].
The NHMRC also included a recommendation for an upper safe intake level set at 3,000 mg/day for children, adolescents, and adults, similar to that established by the US FDA [37]. In establishing this level, they considered evidence that suggested that high levels of these fatty acids may impair immune responses and prolong bleeding times. However, the immune function tests were performed in vitro, and it is unclear how the results would translate to the in vivo situation. Prolonged bleeding times have been detected in Inuit subjects, but it is not known if they were the result of high n-3 LCPUFA consumption levels.
The French Food Safety Authority (ANSES) published in 2011 an extensive report on dietary recommendations for lipids, including detailed recommendations for specific physiological conditions [38]. Based on the low conversion of ALA into DHA, the ANSES recommended a daily intake of 250 mg of DHA to meet adult nutritional requirements, which is twice the recommendation established in 2001 [39]. Similarly, and after considering data regarding risk reduction related to n-3 LCPUFA consumption, a recommendation for adults was established at 500 mg of combined DHA and EPA. For younger age groups the recommended intake levels were similar to those of adults (10–18 years): 70 mg of DHA (1–3 years); 125 mg of DHA and 250 mg of combined DHA and EPA (3–9 years). For pregnant and lactating women, the ANSES recommendations were 250 mg of DHA or 500 mg of combined DHA and EPA.
With regard to the management of NCDs, ANSES reviewed the available evidence and concluded that it was sufficient to establish a minimum daily intake recommendation for the risk reduction of psychiatric diseases, in particular depression (200–300 mg of DHA + EPA). Similarly, for CVD risk reduction in the general population, ANSES recommends a daily intake of 500 mg of combined EPA and DHA. However, this recommendation is increased up to 750 mg/day for subjects with increased cardiovascular risk profiles.
Go to : 
Numerous randomized clinical trials, observational studies, and in vitro and animal studies have investigated the effects of seafood consumption and n-3 LCPUFAs on cardiovascular conditions and diseases. More recently, such data have been compiled in several systematic reviews, metanalyses, and expert opinions regarding the role of n-3 LCPUFA in cardiovascular health [326273031323839404142434445464748495051].
As most currently available data are overall supportive of n-3 LCPUFA reducing the risk of cardiac disease or death, expert guidelines have converged toward the development of consistent recommendations for the general population to consume at least 250 mg/day of n-3 LCPUFA or at least 2 servings/week of oily fish [262730313238]. Despite these recommendations, not all reviews support the presence of a beneficial role of n-3 LCPUFA in cardiovascular health [495051]. Recently it was suggested that the results in clinical trials showing limited to no effect cardiovascular health are likely associated with heterogeneity among populations, n-3 LCPUFA status, and intake discrepancies, as well as some specific questions related to the precise physiological effects and molecular mechanisms that account for the observed benefits, the magnitudes, and dose responses in specific clinical outcomes [52].
However, given the benefits associated with CVD risk reduction, the absence of adverse effects associated with the daily consumption of n-3 LCPUFA at the recommended levels and the overall supportive evidence, it is considered appropriate for all individuals to increase their daily n-3 LCPUFA consumption.
Recent clinical data and nutritional recommendations established by expert authorities as well as findings in ongoing studies are reviewed in the following sections in order to support relevant recommendations regarding n-3 LCPUFA and cardiovascular health.
Data from 2 large human intervention studies, the Vitamin D and Omega-3 Trial (VITAL) [53] and the Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial (REDUCE-IT) [54], were published in late 2018, thereby adding considerable insights on the role of n-3 LCPUFA in CVD and how this association may be translated into dietary recommendations.
The primary conclusion of the VITAL study was not positive regarding the effect of supplementation with n-3 LCPUFA on major cardiovascular events as the data failed to show a lower incidence of major cardiovascular events in the experimental group than in the placebo control group [53]. However, an in-depth analysis of the study results did reveal highly relevant cardiovascular effects following supplementation with n-3 LCPUFA. Indeed, while the primary outcome of the study, a reduction in major cardiovascular events (a composite of myocardial infarction, stroke, and death from cardiovascular causes), was not achieved with statistical significance, the analysis did demonstrate an 8% reduction in such events. However, the secondary outcomes were highly relevant as omega-3 supplementation was associated with a reduction in total and fatal myocardial infarction risk by 28% and 50%, respectively. A 17% risk reduction was recorded for total coronary heart disease, versus placebo. Interestingly for African-Americans, the study found a dramatic 77% reduction in heart attacks linked to daily omega-3 supplementation. In people who do not consume the recommended 1 or 2 servings of fish a week, the n-3 LCPUFA supplementation was linked to a 40% reduction in heart attacks; however, among the subjects who followed the fish consumption recommendation, there was no such association.
The conclusion of the REDUCE-IT study for patients with elevated triglyceride levels, despite the use of statins, the risk of ischemic events, including cardiovascular death, was significantly lower among those who received 2 g of icosapent ethyl (a purified ethyl ester of EPA) twice daily than among those who received the placebo [54]. The data indicated that 4 g/day of EPA reduced the risk of the first incidence of a major adverse cardiovascular events by 25% (including cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, coronary revascularization, or unstable angina requiring hospitalization). Furthermore, a 26% reduction of cardiovascular death (myocardial infarction or stroke) was reported, while the risk of cardiovascular death or non-fatal myocardial infarction were reduced by 25%. Additional risk reductions included: 1) 31% lower risk of fatal or non-fatal myocardial infarction; 2) 20% lower risk of cardiovascular death; 3) 32% lower risk of hospitalization or unstable angina; and 4) 28% lower risk of fatal or non-fatal stroke.
The American Heart Association (AHA) issued a science advisory based on the totality of available data including 2 recent study outcomes [43]. The AHA concluded that prescription of n-3 fatty acids (EPA + DHA or EPA-only) at a dose of 4 g/day (> 3 g/day total EPA + DHA) would be an effective and safe option for reducing triglycerides, either as a monotherapy or as an adjunct to other lipid-lowering agents. Among patients with elevated triglyceride levels, despite the use of statins, the risk of ischemic events, including cardiovascular death, was significantly lower among those who received 2 g of icosapent ethyl twice daily than among those who received the placebo. The suggested use of n-3 LCPUFA (4 g/day) for improving atherosclerotic CVD risk in patients with hypertriglyceridemia is supported by the detection of a 25% reduction in major adverse cardiovascular events in the REDUCE-IT data.
Several expert authorities have issued recommendations regarding n-3 LCPUFA intake. The ANSES established dietary recommendations for the French population that specifically detailed the dietary recommendations for CVD risk reduction within the context of the increasing incidence of NCDs [38]. ANSES concluded that a dietary intake of fatty acids can influence cardiovascular risk by affecting several risk markers and by influencing other parameters associated with atherosclerosis. Studies on the effect of fatty acids rarely investigate fatty acid supplementation, except for n-3 LCPUFA. The relationship between the consumption of fish or EPA/DHA and cardiovascular risk is dependent on the evaluated parameters. Epidemiological and intervention studies have demonstrated that the consumption of fish or EPA and DHA reduces cardiovascular mortality. These effects are observed for dietary intakes between 0.4 g and 1.8 g/day of n-3 LCPUFA. Consequently, a daily dietary intake recommendation of 500 mg of EPA and DHA (or 0.25% of energy intake) is justifiable for the general population within the context of achieving cardiovascular risk reduction. The magnitude of this intake recommendation, which was based on intervention study results, can reach 750 mg/day for subjects with increased cardiovascular risks.
The 2015–2020 Dietary Guidelines for Americans and the Scientific Report of the 2015 US Dietary Guidelines Advisory Committee recommend at least 2 servings of fish per week (providing an average of 250 mg EPA + DHA per day) in place of servings of other animal sources of protein [3233]. The 2015–2020 US Dietary Guidelines for Americans provide information about how to incorporate seafood into the healthy US-style eating pattern and the healthy Mediterranean-style eating pattern.
The AHA recommended, in its 2018 science advisory, one to 2 seafood meals per week to reduce the risk of congestive heart failure, coronary heart disease, ischemic stroke, and sudden cardiac death, especially when seafood replaces the intake of less healthy foods [42].
Go to : 
The roles of n-3 and n-6 LCPUFA in brain and cognitive development during early life have been extensively investigated [555657]. While both LCPUFAs are present in breast milk and have been demonstrated to play an important role in growth and development, the intake of n-3 LCPUFA during pregnancy and early life has been reported to affect growth as well as neurological and immune function in later life [5556575859]. The key role of n-3 LCPUFA has been associated specifically with DHA as it is a key component of membrane structural lipids, especially in nervous tissue and the retina. The developing brain accumulates large amounts of DHA both pre- and postnatally, particularly during the first 2 years of life. Therefore, an adequate DHA status is associated with optimal brain growth and development, and dietary intake recommendations of preformed DHA have been developed for application during pregnancy and infancy.
The role of n-3 LCPUFA in managing brain performance beyond infancy and childhood, and more specifically in brain disorders or disease, has become a markedly expanding research topic over the last 2 decades and will be discussed in the following sections.
A role of n-3 LCPUFA in neurodegenerative disorders is based on observations that several brain functions are influenced by n-3 LCPUFA, an important constituent of the plasma membrane and implicated in different processes, including increased synaptic development and functionality [60], effects on synaptic integrity and plasticity [61626364], contributions to neuroplasticity and subsequent enhancement of cognitive activity [65].
There is recent evidence that n-3 LCPUFA supplementation may have beneficial effects on neurodegenerative disorders [6667], such as Parkinson's disease (PD) and Alzheimer's disease (AD) [68697071]. Although the available data are at present non-conclusive, dietary recommendations for n-3 LCPUFA supplementation in patients with PD or AD may alleviate some of the disease symptoms or slow the related cognitive and physical declines.
Some, but not all, observational studies have suggested that diets that are high in n-3 LCPUFA are associated with reduced risks of cognitive decline, AD, or dementia [7273]. Because DHA is an essential component of cellular membrane phospholipids in the brain, researchers have hypothesized that n-3 LCPUFAs might protect cognitive function by helping to maintain neuronal function and cell membrane integrity within the brain [73]. This hypothesis is supported by results from case-control studies indicating that patients with AD have lower serum levels of DHA than cognitively healthy people [7475]. Lower serum DHA levels have also been associated with an increase in cerebral amyloidosis (build-up of protein deposits called amyloids) in healthy older adults, whereas a high DHA level has been associated with preservation of brain volume [76].
There have been several observational studies on the effects of fish, EPA, and/or DHA intake on cognitive function in healthy older adults. Fish consumption has been associated with less cognitive decline at a 5-year follow-up in a prospective cohort study involving 210 healthy men aged 70–89 years [77]. The study also reported a dose-response relationship between tertiles of dietary EPA + DHA intake and subsequent 5-year cognitive decline. Similarly, relatively high fish consumption among 5,386 study participants was associated with a 60% lower risk of dementia and a 70% lower risk of AD over an average of 2.1 years in a population-based prospective study of people aged 55 or older who were free from dementia at baseline [78]. However, the 6-year follow-up did not detect associations between omega-3 intake and incidence of dementia or AD [79], a result the authors explained was due to the short follow-up period in the first analysis and the small number of patients who developed dementia. A higher omega-3 index was associated with a greater hippocampal volume in the Women's Health Initiative Memory Study [80] and with a larger brain volume and improved cognitive test scores in the Framingham offspring cohort-based study [81]. In a 2016 dose-response meta-analysis of 21 cohort studies, the authors reported that increased intakes of fish or dietary DHA were inversely associated with the risks of dementia and AD [82]. Specifically, a 100 mg/day incremental increase in DHA intake was associated with a 14% lower risk of dementia and a 37% lower risk of AD.
Clinical studies, on the contrary, are notably less conclusive than observational studies, and they do not report a beneficial effect of n-3 LCPUFA supplementation on cognitive function in older adults with no cognitive impairment. In a trial in the United Kingdom, 748 cognitively healthy adults aged 70–79 years received either 500 mg DHA and 200 mg EPA or a placebo daily for 24 months [83]. Cognitive function did not differ significantly between the 2 groups, although cognitive function did not decline in either group. In the AREDS2 study, dietary intervention with 350 mg DHA and 650 mg EPA for 5 years did not have a significant effect on cognitive function in 3,501 older adults (mean age 72.7 years) with age-related macular degeneration [74]. Clinical trial results have also suggested that n-3 LCPUFA supplementation does not appear to benefit patients with AD, although it might benefit patients with mild cognitive impairment. For example, compared to the placebo results, daily supplementation with 2 g DHA for 18 months did not slow the rate of cognitive decline in 295 participants (mean age 76 years) with mild to moderate AD [84]. In the OmegaAD trial, daily supplementation with 1,700 mg DHA and 600 mg EPA for 6 months in 174 older adults with mild to moderate AD also failed to slow down the rate of cognitive decline from that obtained in the placebo group [85]. However, a subgroup of patients with very mild impairment experienced a significant reduction in the rate of cognitive decline. In a small trial (35 older adults with mild cognitive impairment) in Malaysia, fish oil supplementation (1,290 mg DHA and 450 mg EPA daily) for 12 months improved memory—particularly short-term, working, and verbal memory—and delayed recall compared to that in the placebo group [86].
Several systematic reviews and meta-analyses, including a Cochrane review, have assessed the effects of n-3 LCPUFA supplementation on cognitive function and dementia in healthy older adults and those with AD or cognitive impairment [878889]. Overall, the findings indicate that n-3 LCPUFA supplementation does not have a different effect on cognitive function in healthy older adults or in people with AD than that from placebo treatment. However, for people with mild cognitive impairment, n-3 LCPUFA may improve certain aspects of cognitive function, including attention, processing speed, and immediate recall [89].
A recent systematic review on the effects of n-3 LCPUFA supplementation on cognitive function in patients with PD or AD also reported an inconsistency between the evidence from prospective observational studies and randomized trials, and the authors suggested some potential reasons for the inconsistency [90]. The authors highlighted that inconsistent results are not unusual in clinical research between observational and randomized studies, particularly when investigating treatments with dietary supplements or integrators. Some of the reasons that may account for the inconsistencies are that, in controlled trials, dietary supplementation is usually carried out over a relatively limited time span compared with the life-long exposure of real-life observational studies; thus, a difference in the time courses of the 2 approaches could play a relevant role. Observational studies may disclose preventive effects associated with disease initiation, whereas in randomized trials involving patients already carrying a disease, the outcome more likely presents as a slowing of disease progression or a reduction in disease-related complications. Moreover, distinct protective mechanisms are likely to be activated. Furthermore, variations in dietary patterns might reflect the adoption of a healthier lifestyle, adopted in concert with the contribution provided by the single-nutrient supplementation. This was postulated, for instance, when investigating the protective effects of the Mediterranean diet on cognitive performances. In the present context, the intake of larger amounts of foods that contain omega-3 fatty acids might be associated with the reduced intake of other nutrients, such as from other types of meat.
Finally, the possibility of different individual responses to dietary intervention must be considered. Avallone and colleagues [90] stated that the protective effects exerted by n-3 LCPUFAs are likely to be modulated by patient-related factors, some of which may have a significant genetic component and may, therefore, be unmodifiable, and furthermore, may be unpredictable via routine clinical and biochemical evaluation. Regardless, treatment with omega-3 fatty acids is generally reported to be safe and well-tolerated. Therefore, Avallone and colleagues [90] concluded n-3 LCPUFA supplementation may indeed be a valuable and biologically plausible tool in the management of neurodegenerative diseases. Nonetheless, supplementation should be part of a global lifestyle intervention and should be undertaken in the early stages of a disease, when a benefit may be more easily detected. Hopefully, the adoption of personalized treatment strategies, aimed to predict individual responses, will help to optimize the effectiveness of such interventions. Such approaches may assist in reducing the current progressive rise in neurodegenerative disorders.
Major depressive disorder (MDD) affects one tenth of the world's population and has been reported to be the world's leading cause of disability [9192]. MDD is of heterogeneous etiology with multiple contributory biological mechanisms. Pharmacological treatments with the currently available antidepressants, although proven to be effective in treating moderate to severe symptoms in MDD, have modest beneficial effects and various adverse effects [93]. Therefore, to optimize patients' outcomes, clinicians need more efficacious and tolerable treatments that have been supported by evidence from thorough scientific investigation and are based on reliable practice guidelines.
The n-3 LCPUFA components EPA and DHA have drawn the clinical attention of medical specialists [94959697]. Several lines of evidence have suggested the efficacy of n-3 LCPUFA as a MDD risk reduction or dietary management strategy in epidemiological and case-controlled studies [9899], randomized-controlled trials [100101102103104105106107108109110], and meta-analyses [111112113114115116117118119120]. In addition to clinical studies that have examined efficacy [121122] and tolerability [123], the mechanisms of the n-3 LCPUFA antidepressant effects have also been thoroughly studied. Several key mechanisms have been proposed, including neuronal cell plasticity and neurogenesis, neurotransmitter dysregulation, and neuro-inflammation [95124125].
Evidence regarding the effectiveness of n-3 LCPUFA in depression is compelling, but not all results are conclusive. Indeed, a 2016 meta-analysis of 26 studies found a 17% lower risk of depression associated with a higher fish intake [126]. A 2015 Cochrane review of 26 studies, on the contrary, found insufficient evidence to determine whether n-3 LCPUFA supplementation (doses ranging from 1,000 to 6,600 mg/day EPA, DHA, and/or other n-3 LCPUFA) are beneficial in MDD in adults [120]. However, those authors did report a small-to-modest beneficial effect on depressive symptoms, although that effect was not clinically significant. A 2019 meta-analysis of double-blind randomized placebo-controlled trials (including 26 studies; 2,160 participants) showed an overall beneficial effect of n-3 LCPUFA on depression symptoms, and both EPA-pure (= 100% EPA) and EPA-major formulations (≥ 60% EPA) demonstrated clinical benefits at an EPA dosage of ≤ 1 g/day [127]. DHA-pure and DHA-major formulations, on the contrary, did not exhibit such benefits. The results of this meta-analysis support the finding that n-3 LCPUFA with EPA ≥ 60% at a dosage of ≤ 1 g/day could have beneficial effects on depression. The authors recommended that further studies examining supplementation with n-3 LCPUFA for specific subgroups of subjects with differing inflammation status and severity of depression are warranted to elucidate the dose responses for both EPA and DHA supplementation.
Most recently, the International Society for Nutritional Psychiatry Research organized an expert panel to conduct a literature review and a Delphi process to develop consensus-based practice guidelines for clinical use of n–3 LCPUFA in MDD [128]. The guidelines indicate that both pure EPA or an EPA/DHA combination of a ratio higher than 2 (EPA/DHA > 2) are considered effective at a recommended dosage of 1–2 g of net EPA daily, from either pure EPA or an EPA/DHA (> 2:1) formula. The guidelines further indicate that the quality of the n-3 LCPUFA may affect therapeutic activity, and that potential adverse effects, such as gastrointestinal and dermatological conditions and comprehensive metabolic panel results, should be monitored. The expert panel agreed on the suitability of using n-3 LCPUFA in MDD treatment for pregnant women, children, and the elderly, and for MDD prevention in high-risk populations. Finally, they suggested that personalizing the clinical application of n-3 LCPUFA in subgroups of MDD with a low omega-3 index or with high levels of inflammatory markers can be regarded as areas that deserve further research.
The ANSES considered, in their 2011 report, that the totality of studies regarding n-3 LCPUFA demonstrate that it is associated with psychopathological processes, and its dietary intakes are capable of influencing those processes [38]. Moreover, ANSES concluded that, based on the totality of available data, there is plausible evidence supporting recommendations for a sufficient dietary intake of PUFA, and in particular n-3 LCPUFA for support maintenance in mental health. With regard to preventing psychiatric disorders, specifically depression, current data enabled ANSES to propose a dietary intake recommendation for n-3 LCPUFA of at least 200–300 mg/day.
Go to : 
As highlighted previously, n-3 LCPUFA have been extensively studied for their roles in brain and cognitive development during early life [555657]. More recently interest in the role of n-3 LCPUFA during early life has increased as there is growing evidence that they can improve pregnancy outcomes, such as gestation duration, and because they are also believed to increase infant growth and enhance short- and long-term development of the offspring [129]. Two recent clinical trials and a meta-analysis have indicated the beneficial effect of n-3 LCPUFA on risk reduction associated with premature birth when provided at a daily intake of 600–800 mg of DHA-rich algal oil or DHA + EPA-rich fish oil [130131].
Data from a double-blind, multicenter, randomized, and controlled intervention trial involving 2,399 women who, during the last trimester of pregnancy, received either DHA-rich fish oil capsules (providing 800 mg/day of DHA) or a matched vegetable oil capsules without DHA demonstrated a reduced incidence of premature birth in the DHA group [130]. Moreover, fewer early-preterm births (< 34 weeks' gestation) were reported in the DHA group compared with the control group (1.09% vs. 2.25%; P = 0.03). Duration of gestation was higher in the DHA group than in the control group (17.59% vs. 13.72%; P = 0.01) and there was a higher mean birth weight (+ 68 g) and fewer low birth weight infants (3.41% vs. 5.27%; P = 0.03) in the DHA group. However, mean birth weight z scores (corrected for gestational age and sex) did not differ between groups, indicating that group differences in birth size were largely a function of gestational age at birth.
Data from another recent double-blind, randomized, and controlled intervention trial involving 530 women who, from 20 weeks of gestation till birth, received either a DHA-rich algal oil capsule (providing 600 mg/day of DHA) or a matched vegetable oil capsules without DHA also demonstrated reduced incidence of premature birth in the DHA group [131]. In addition, the data demonstrated fewer infants born at < 34 weeks of gestation in the DHA-supplemented group compared to that in the placebo group (P = 0.025). DHA supplementation also resulted in longer gestation duration (2.9 day; P = 0.041), greater birth weight (172 g; P = 0.004), birth length (0.7 cm; P = 0.022), and head circumference (0.5 cm; P = 0.012). Preterm infants born in the DHA-supplemented group had a shorter hospital stay than that in the placebo group (8.9 vs. 40.8 days; P = 0.026). The mean DHA intake for the DHA-supplemented group was 469 mg/day, resulting in higher maternal and cord RBC-phospholipid-DHA levels (2.6%; P < 0.001) than those in the placebo group. There were no reported adverse effects and no safety concerns were identified in the DHA group.
A recent meta-analysis, published in late 2018, included 70 randomized-controlled trials (19,927 women at low, mixed or high risk of poor pregnancy outcomes) and compared n-3 LCPUFA interventions (supplements and food) with placebo or non-n-3 LCPUFA treatments. The authors reported that both preterm births (< 37 weeks) and early-preterm births (< 34 weeks) were reduced in women receiving n-3 LCPUFA treatments [5]. Additionally, the authors detected a possible reduced risk of perinatal death, neonatal care admission, and low birth weight infants as well as a small increased risk of low gestational age infants with n-3 LCPUFA supplementation. The authors concluded that n-3 LCPUFA supplementation during pregnancy is an effective strategy for reducing the incidence of preterm births; however, it probably increases the incidence of post-term pregnancies. They also suggested that more studies comparing n-3 LCPUFA and placebo treatments to establish causality in relation to preterm births are not needed.
DHA supplementation during pregnancy was estimated to be both medically and socio-economically relevant through its ability to reduce the incidence of premature birth [132]. Shireman and colleagues [132] demonstrated, after applying a post-hoc cost analysis of delivery hospitalizations and all hospitalizations in the year following deliveries at the Kansas University Hospital, that hospital cost savings related to DHA supplementation were estimated to be USD 1,678 per infant. Even after adjusting for the estimated cost of providing 600 mg/day of supplemental DHA for 26 weeks (USD 166.48) and a slightly higher maternal care cost (USD 26) in the DHA group, the net saving per mother-child pair was USD 1,484. Extrapolating this to the nearly 4 million US deliveries per year suggests that universal supplementation with 600 mg/day during the last 2 trimesters of pregnancy could save the US health care system up to USD 6 billion.
In an effort to assist in the development of implementation strategies to increase pregnant women's n-3 LCPUFA intake, a recent study assessed the awareness of Australian pregnant women about preterm birth, their nutrition and supplementation behaviors during pregnancy, and their intentions to increase n-3 fatty acid intake [133]. The authors of the study indicated that the main information source for women about preterm birth and dietary supplementation recommendations during pregnancy is their health professional. Therefore, informing women about ways to reduce the risk of preterm birth, including the role of n-3 LCPUFA, should occur during antenatal visits. Those results may be useful to clinicians caring for pregnant women and for the next stage of translation of the Cochrane review findings—the design of implementation strategies to increase the intake of n-3 LCPUFA during pregnancy where needed.
A recent overview of the lipid requirements of preterm infants emphasizes the importance of an adequate dietary intake of n-3 LCPUFA to support normal development of preterm infants [6]. Lapillonne [6] highlighted that current nutritional management guidelines may not be suitable for recommending sufficient amounts of preformed DHA. Based on available data, Koletzko and colleagues [134] established daily recommended intakes of 55–60 mg/kg of DHA, < 20 mg mg/kg of EPA, and 35–45 mg/day of arachidonic acid (ARA) to meet the nutritional needs of fully enterally fed preterm infants.
Most recently, there is renewed attention being paid to the recommendations for DHA and infant nutrition, which was triggered by a recently adopted regulatory standards on infant and follow-on formula for the European Union. Those standards stipulate that from February 2020 onwards, all such products marketed in the European Union must contain 20–50 mg DHA per 100 kcal, which is equivalent to about 0.5–1% of fatty acids (higher than that typically found in human milk and current infant formula products) without the need to also include ARA (20:4n-6) [7]. This novel approach to infant formula composition has given rise to concern and controversy because there is no accountable evidence source for the standards' suitability and safety in healthy infants. Therefore, a group of international experts in the field of infant nutrition recently published a review of the state of scientific research on DHA and ARA, and addressed the controversies arising from the new European regulatory standards. They concluded that based on the available information, infant and follow-on formula should provide both DHA and ARA. The DHA should equal at least the mean content in human milk globally (0.3% of FAs) but preferably reach a level of 0.5% of FAs. Although the optimal ARA intake amounts remain to be defined, the experts strongly recommended that ARA should be provided along with DHA. At DHA amount in infant formula of up to 0.64%, ARA contents should at least equal the DHA contents. Finally, the experts concluded that further well-designed clinical studies should evaluate the optimal intakes of DHA and ARA in infants at different ages and those evaluations should be based on relevant outcomes.
Go to : 
Notes
Conflict of Interest: The author is an employee of DSM Nutritional Products, a supplier of nutritional solutions and ingredients including n-3 long-chain polyunsaturated fatty acid.
Go to : 
References
1. World Health Organization (WHO). Global Status Report on Noncommunicable Diseases 2014. Geneva: WHO;2014.
2. World Health Organization (WHO). Global Action Plan for the Prevention and Control of NCDs 2013–2020 [Internet]. Geneva: WHO;2013. cited 2020 April 5. Available from: https://www.who.int/nmh/publications/ncd-action-plan/en/.
3. Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011; 58:2047–2067. PMID: 22051327.
4. Sinn N, Milte CM, Street SJ, Buckley JD, Coates AM, Petkov J, Howe PR. Effects of n-3 fatty acids, EPA v. DHA, on depressive symptoms, quality of life, memory and executive function in older adults with mild cognitive impairment: a 6-month randomised controlled trial. Br J Nutr. 2012; 107:1682–1693. PMID: 21929835.

5. Middleton P, Gomersall JC, Gould JF, Shepherd E, Olsen SF, Makrides M. Omega-3 fatty acid addition during pregnancy. Cochrane Database Syst Rev. 2018; 11:CD003402. PMID: 30480773.

6. Lapillonne A. Enteral and parenteral lipid requirements of preterm infants. World Rev Nutr Diet. 2014; 110:82–98. PMID: 24751623.

7. Koletzko B, Bergmann K, Brenna JT, Calder PC, Campoy C, Clandinin MT, Colombo J, Daly M, Decsi T, Demmelmair H, Domellöf M, FidlerMis N, Gonzalez-Casanova I, van Goudoever JB, Hadjipanayis A, Hernell O, Lapillonne A, Mader S, Martin CR, Matthäus V, Ramakrishan U, Smuts CM, Strain SJ, Tanjung C, Tounian P, Carlson SE. Should formula for infants provide arachidonic acid along with DHA? A position paper of the European Academy of Paediatrics and the Child Health Foundation. Am J Clin Nutr. 2020; 111:10–16. PMID: 31665201.

8. Goyens PL, Spilker ME, Zock PL, Katan MB, Mensink RP. Conversion of alpha-linolenic acid in humans is influenced by the absolute amounts of alpha-linolenic acid and linoleic acid in the diet and not by their ratio. Am J Clin Nutr. 2006; 84:44–53. PMID: 16825680.
9. Kinsella JE, Lokesh B, Broughton S, Whelan J. Dietary polyunsaturated fatty acids and eicosanoids: potential effects on the modulation of inflammatory and immune cells: an overview. Nutrition. 1990; 6:24–44. PMID: 2135755.
10. Clandinin MT. Brain development and assessing the supply of polyunsaturated fatty acid. Lipids. 1999; 34:131–137. PMID: 10102239.

11. Salem N Jr, Wegher B, Mena P, Uauy R. Arachidonic and docosahexaenoic acids are biosynthesized from their 18-carbon precursors in human infants. Proc Natl Acad Sci U S A. 1996; 93:49–54. PMID: 8552667.

12. Schaeffer L, Gohlke H, Müller M, Heid IM, Palmer LJ, Kompauer I, Demmelmair H, Illig T, Koletzko B, Heinrich J. Common genetic variants of the FADS1 FADS2 gene cluster and their reconstructed haplotypes are associated with the fatty acid composition in phospholipids. Hum Mol Genet. 2006; 15:1745–1756. PMID: 16670158.

13. Micha R, Khatibzadeh S, Shi P, Fahimi S, Lim S, Andrews KG, Engell RE, Powles J, Ezzati M, Mozaffarian D. Global Burden of Diseases Nutrition and Chronic Diseases Expert Group NutriCoDE. Global, regional, and national consumption levels of dietary fats and oils in 1990 and 2010: a systematic analysis including 266 country-specific nutrition surveys. BMJ. 2014; 348:g2272. PMID: 24736206.

14. Patterson AC, Hogg RC, Kishi DM, Stark KD. Biomarker and dietary validation of a Canadian food frequency questionnaire to measure eicosapentaenoic and docosahexaenoic acid intakes from whole food, functional food, and nutraceutical sources. J Acad Nutr Diet. 2012; 112:1005–1014. PMID: 22583924.

15. Fratesi JA, Hogg RC, Young-Newton GS, Patterson AC, Charkhzarin P, Block Thomas K, Sharratt MT, Stark KD. Direct quantitation of omega-3 fatty acid intake of Canadian residents of a long-term care facility. Appl Physiol Nutr Metab. 2009; 34:1–9. PMID: 19234579.

16. Patterson AC, Metherel AH, Hanning RM, Stark KD. The percentage of DHA in erythrocytes can detect non-adherence to advice to increase EPA and DHA intakes. Br J Nutr. 2014; 111:270–278. PMID: 23920312.

17. Block RC, Harris WS, Pottala JV. Determinants of blood cell omega-3 fatty acid content. Open Biomark J. 2008; 1:1–6. PMID: 19953197.
18. Flock MR, Skulas-Ray AC, Harris WS, Etherton TD, Fleming JA, Kris-Etherton PM. Determinants of erythrocyte omega-3 fatty acid content in response to fish oil supplementation: a dose-response randomized controlled trial. J Am Heart Assoc. 2013; 2:e000513. PMID: 24252845.

19. Stark KD, Beblo S, Murthy M, Whitty JE, Buda-Abela M, Janisse J, Rockett H, Martier SS, Sokol RJ, Hannigan JH, Salem N Jr. Alcohol consumption in pregnant, black women is associated with decreased plasma and erythrocyte docosahexaenoic acid. Alcohol Clin Exp Res. 2005; 29:130–140. PMID: 15654301.

20. Harris WS, Pottala JV, Lacey SM, Vasan RS, Larson MG, Robins SJ. Clinical correlates and heritability of erythrocyte eicosapentaenoic and docosahexaenoic acid content in the Framingham Heart Study. Atherosclerosis. 2012; 225:425–431. PMID: 22727409.

21. Crowe FL, Skeaff CM, Green TJ, Gray AR. Serum n-3 long-chain PUFA differ by sex and age in a population-based survey of New Zealand adolescents and adults. Br J Nutr. 2008; 99:168–174. PMID: 17678566.

22. Siscovick DS, Raghunathan TE, King I, Weinmann S, Wicklund KG, Albright J, Bovbjerg V, Arbogast P, Smith H, Kushi LH, Cobb LA, Copass MK, Psaty BM, Lemaitre R, Retzlaff B, Childs M, Knopp RH. Dietary intake and cell membrane levels of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. JAMA. 1995; 274:1363–1367. PMID: 7563561.

23. Albert CM, Campos H, Stampfer MJ, Ridker PM, Manson JE, Willett WC, Ma J. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N Engl J Med. 2002; 346:1113–1118. PMID: 11948270.

24. Schaefer EJ, Bongard V, Beiser AS, Lamon-Fava S, Robins SJ, Au R, Tucker KL, Kyle DJ, Wilson PW, Wolf PA. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: the Framingham Heart Study. Arch Neurol. 2006; 63:1545–1550. PMID: 17101822.
25. Stark KD, Van Elswyk ME, Higgins MR, Weatherford CA, Salem N Jr. Global survey of the omega-3 fatty acids, docosahexaenoic acid and eicosapentaenoic acid in the blood stream of healthy adults. Prog Lipid Res. 2016; 63:132–152. PMID: 27216485.

26. Food and Agriculture Organization of the United Nations. FAO Fats and Fatty Acids in Human Nutrition. Report of an Expert Consultation. Rome: Food and Agriculture Organization of the United Nations;2010.
27. EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010; 8:1461.
28. EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the Tolerable Upper Intake Level of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and docosapentaenoic acid (DPA). EFSA J. 2012; 10:2815.
29. Institute of Medicine (US). Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, D.C.: National Academy Press;2005.
30. U.S. Food and Drug Administration (FDA). Advice about Eating Fish [Internet]. Silver Spring: FDA;2020. cited 2020 April 5. Available from: https://www.fda.gov/food/consumers/advice-about-eating-fish.
31. U.S. Food and Drug Administration (FDA). Qualified Health Claims: Letters of Enforcement Discretion [Internet]. Silver Spring: FDA;2020. cited 2020 April 5. Available from: https://www.fda.gov/food/food-labeling-nutrition/qualified-health-claims-letters-enforcement-discretion.
32. U.S. Department of Health and Human Services. U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. 8th ed. Washington, D.C: U.S. Government Printing Office;2015.
33. US Department of Agriculture. US Department of Health and Human Services. Scientific Report of the 2015 Dietary Guidelines Advisory Committee. Washington, D.C.: U.S. Government Printing Office;2015.
34. National Health and Medical Research Council (AU). Nutrient Reference Values for Australia and New Zealand including Recommended Dietary Intakes (version 1.2). Canberra: National Health and Medical Research Council (AU);2017.
35. Dolecek TA. Epidemiological evidence of relationships between dietary polyunsaturated fatty acids and mortality in the multiple risk factor intervention trial. Proc Soc Exp Biol Med. 1992; 200:177–182. PMID: 1579579.

36. Iso H, Rexrode KM, Stampfer MJ, Manson JE, Colditz GA, Speizer FE, Hennekens CH, Willett WC. Intake of fish and omega-3 fatty acids and risk of stroke in women. JAMA. 2001; 285:304–312. PMID: 11176840.

37. Department of Health and Human Services, U.S. Food and Drug Administration. Substances affirmed as generally recognized as safe: menhaden oil [Internet]. College Park: Office of the Federal Register;2005. cited 2020 April 5. https://www.federalregister.gov/documents/2005/03/23/05-5641/substances-affirmed-as-generally-recognized-as-safe-menhaden-oil.
38. French Agency for Food, Environmental and Occupational Health Safety (ANSES). Update of the Recommended Nutritional Intake for Fatty Acids. Collective Expert Report [Internet]. Maisons-Alfort: ANSES;2011. cited 2020 April 5. Available from: https://www.anses.fr/en/system/files/NUT2006sa0359Ra.pdf.
39. Beaufrère B, Bresson JL, Briend A, Ghisolfi J, Goulet A, Putet G, Rieu D, Turck D, Vidailhet M. Martin A, editor. Recommended Nutritional Intakes for the French Population. Paris: Ed Tec et Doc.;2001.
40. Mozaffarian D, Wu JH. (n-3) fatty acids and cardiovascular health: are effects of EPA and DHA shared or complementary? J Nutr. 2012; 142:614S–625S. PMID: 22279134.

41. Kris-Etherton PM, Harris WS, Appel LJ; Nutrition Committee. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002; 106:2747–2757. PMID: 12438303.

42. Siscovick DS, Barringer TA, Fretts AM, Wu JH, Lichtenstein AH, Costello RB, Kris-Etherton PM, Jacobson TA, Engler MB, Alger HM, Appel LJ, Mozaffarian D. American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health. Council on Epidemiology and Prevention. Council on Cardiovascular Disease in the Young. Council on Cardiovascular and Stroke Nursing. Council on Clinical Cardiology. Omega-3 polyunsaturated fatty acid (fish oil) supplementation and the prevention of clinical cardiovascular disease: a science advisory from the American Heart Association. Circulation. 2017; 135:e867–e884. PMID: 28289069.

43. Skulas-Ray AC, Wilson PWF, Harris WS, Brinton EA, Kris-Etherton PM, Richter CK, Jacobson TA, Engler MB, Miller M, Robinson JG, Blum CB, Rodriguez-Leyva D, de Ferranti SD, Welty FK. American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology. Council on Lifestyle and Cardiometabolic Health. Cardiovascular Disease in the Young. Council on Cardiovascular and Stroke Nursing. Council on Clinical Cardiology. Omega-3 fatty acids for the management of hypertriglyceridemia: a science advisory from the American Heart Association. Circulation. 2019; 140:e673–91. PMID: 31422671.

44. Aung T, Halsey J, Kromhout D, Gerstein HC, Marchioli R, Tavazzi L, Geleijnse JM, Rauch B, Ness A, Galan P, Chew EY, Bosch J, Collins R, Lewington S, Armitage J, Clarke R. Omega-3 Treatment Trialists' Collaboration. Associations of omega-3 fatty acid supplement use with cardiovascular disease risks: meta-analysis of 10 trials involving 77,917 individuals. JAMA Cardiol. 2018; 3:225–234. PMID: 29387889.
45. Abdelhamid AS, Brown TJ, Brainard JS, Biswas P, Thorpe GC, Moore HJ, Deane KH, AlAbdulghafoor FK, Summerbell CD, Worthington HV, Song F, Hooper L. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2018; 7:CD003177. PMID: 30019766.

46. Arca M, Borghi C, Pontremoli R, De Ferrari GM, Colivicchi F, Desideri G, Temporelli PL. Hypertriglyceridemia and omega-3 fatty acids: Their often overlooked role in cardiovascular disease prevention. Nutr Metab Cardiovasc Dis. 2018; 28:197–205. PMID: 29397253.

47. Rimm EB, Appel LJ, Chiuve SE, Djoussé L, Engler MB, Kris-Etherton PM, Mozaffarian D, Siscovick DS, Lichtenstein AH. American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health. Council on Epidemiology and Prevention. Council on Cardiovascular Disease in the Young. Council on Cardiovascular and Stroke Nursing. Council on Clinical Cardiology. Seafood long-chain n-3 polyunsaturated fatty acids and cardiovascular disease: a science advisory from the American Heart Association. Circulation. 2018; 138:e35–47. PMID: 29773586.

48. Pizzini A, Lunger L, Sonnweber T, Weiss G, Tancevski I. The role of omega-3 fatty acids in the setting of coronary artery disease and COPD: a review. Nutrients. 2018; 10:1864.

49. Wen YT, Dai JH, Gao Q. Effects of omega-3 fatty acid on major cardiovascular events and mortality in patients with coronary heart disease: a meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2014; 24:470–475. PMID: 24472636.

50. Kwak SM, Myung SK, Lee YJ, Seo HG. Korean Meta-analysis Study Group. Efficacy of omega-3 fatty acid supplements (eicosapentaenoic acid and docosahexaenoic acid) in the secondary prevention of cardiovascular disease: a meta-analysis of randomized, double-blind, placebo-controlled trials. Arch Intern Med. 2012; 172:686–694. PMID: 22493407.
51. Nestel P, Clifton P, Colquhoun D, Noakes M, Mori TA, Sullivan D, Thomas B. Indications for omega-3 long chain polyunsaturated fatty acid in the prevention and treatment of cardiovascular disease. Heart Lung Circ. 2015; 24:769–779. PMID: 25936871.

52. Meyer BJ, Groot RH. Effects of omega-3 long chain polyunsaturated fatty acid supplementation on cardiovascular mortality: the importance of the dose of DHA. Nutrients. 2017; 9:1305.

53. Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, Gibson H, Albert CM, Gordon D, Copeland T, D'Agostino D, Friedenberg G, Ridge C, Bubes V, Giovannucci EL, Willett WC, Buring JE. VITAL Research Group. Marine n−3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med. 2019; 380:23–32. PMID: 30415637.

54. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT Jr, Juliano RA, Jiao L, Granowitz C, Tardif JC, Ballantyne CM. REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019; 380:11–22. PMID: 30415628.

55. Uauy R, Hoffman DR, Peirano P, Birch DG, Birch EE. Essential fatty acids in visual and brain development. Lipids. 2001; 36:885–895. PMID: 11724460.

56. Uauy R, Mena P, Rojas C. Essential fatty acids in early life: structural and functional role. Proc Nutr Soc. 2000; 59:3–15. PMID: 10828169.

57. Carlson SE, Colombo J. Docosahexaenoic acid and arachidonic acid nutrition in early development. Adv Pediatr. 2016; 63:453–471. PMID: 27426911.

58. Makrides M, Gibson RA. Long-chain polyunsaturated fatty acid requirements during pregnancy and lactation. Am J Clin Nutr. 2000; 71:307S–311S. PMID: 10617987.

59. Sattar N, Berry C, Greer IA. Essential fatty acids in relation to pregnancy complications and fetal development. Br J Obstet Gynaecol. 1998; 105:1248–1255. PMID: 9883915.

60. Cansev M, Wurtman RJ, Sakamoto T, Ulus IH. Oral administration of circulating precursors for membrane phosphatides can promote the synthesis of new brain synapses. Alzheimers Dement. 2008; 4:S153–68. PMID: 18631994.

61. Beltz BS, Tlusty MF, Benton JL, Sandeman DC. Omega-3 fatty acids upregulate adult neurogenesis. Neurosci Lett. 2007; 415:154–158. PMID: 17240063.

62. Kawakita E, Hashimoto M, Shido O. Docosahexaenoic acid promotes neurogenesis in vitro and in vivo
. Neuroscience. 2006; 139:991–997. PMID: 16527422.
63. Cutuli D. Functional and structural benefits induced by omega-3 polyunsaturated fatty acids during aging. Curr Neuropharmacol. 2017; 15:534–542. PMID: 27306037.

64. Aryal S, Hussain S, Drevon CA, Nagelhus E, Hvalby Ø, Jensen V, Walaas SI, Davanger S. Omega-3 fatty acids regulate plasticity in distinct hippocampal glutamatergic synapses. Eur J Neurosci. 2019; 49:40–50. PMID: 30367533.

65. Castro-Gómez P, Garcia-Serrano A, Visioli F, Fontecha J. Relevance of dietary glycerophospholipids and sphingolipids to human health. Prostaglandins Leukot Essent Fatty Acids. 2015; 101:41–51. PMID: 26242691.

66. Cardoso C, Afonso C, Bandarra NM. Dietary DHA and health: cognitive function ageing. Nutr Res Rev. 2016; 29:281–294. PMID: 27866493.

67. Calviello G, Su HM, Weylandt KH, Fasano E, Serini S, Cittadini A. Experimental evidence of ω-3 polyunsaturated fatty acid modulation of inflammatory cytokines and bioactive lipid mediators: their potential role in inflammatory, neurodegenerative, and neoplastic diseases. Biomed Res Int. 2013; 2013:743171. PMID: 23691510.
68. Moore K, Hughes CF, Ward M, Hoey L, McNulty H. Diet, nutrition and the ageing brain: current evidence and new directions. Proc Nutr Soc. 2018; 77:152–163. PMID: 29316987.

69. Yassine HN, Braskie MN, Mack WJ, Castor KJ, Fonteh AN, Schneider LS, Harrington MG, Chui HC. Association of docosahexaenoic acid supplementation with Alzheimer disease stage in apolipoprotein E ε4 carriers: a review. JAMA Neurol. 2017; 74:339–347. PMID: 28114437.
70. Butler M, Nelson VA, Davila H, Ratner E, Fink HA, Hemmy LS, McCarten JR, Barclay TR, Brasure M, Kane RL. Over-the-counter supplement interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med. 2018; 168:52–62. PMID: 29255909.
71. Burckhardt M, Herke M, Wustmann T, Watzke S, Langer G, Fink A. Omega-3 fatty acids for the treatment of dementia. Cochrane Database Syst Rev. 2016; 4:CD009002. PMID: 27063583.

72. Dangour AD, Whitehouse PJ, Rafferty K, Mitchell SA, Smith L, Hawkesworth S, Vellas B. B-vitamins and fatty acids in the prevention and treatment of Alzheimer's disease and dementia: a systematic review. J Alzheimers Dis. 2010; 22:205–224. PMID: 20847412.

73. Sydenham E, Dangour AD, Lim WS. Omega 3 fatty acid for the prevention of cognitive decline and dementia. Cochrane Database Syst Rev. 2012; 6:CD005379.

74. Chew EY, Clemons TE, Agrón E, Launer LJ, Grodstein F, Bernstein PS. Age-Related Eye Disease Study 2 (AREDS2) Research Group. Effect of omega-3 fatty acids, lutein/zeaxanthin, or other nutrient supplementation on cognitive function: the AREDS2 randomized clinical trial. JAMA. 2015; 314:791–801. PMID: 26305649.
75. Tully AM, Roche HM, Doyle R, Fallon C, Bruce I, Lawlor B, Coakley D, Gibney MJ. Low serum cholesteryl ester-docosahexaenoic acid levels in Alzheimer's disease: a case-control study. Br J Nutr. 2003; 89:483–489. PMID: 12654166.

76. Yassine HN, Feng Q, Azizkhanian I, Rawat V, Castor K, Fonteh AN, Harrington MG, Zheng L, Reed BR, DeCarli C, Jagust WJ, Chui HC. Association of serum docosahexaenoic acid with cerebral amyloidosis. JAMA Neurol. 2016; 73:1208–1216. PMID: 27532692.

77. van Gelder BM, Tijhuis M, Kalmijn S, Kromhout D. Fish consumption, n-3 fatty acids, and subsequent 5-y cognitive decline in elderly men: the Zutphen Elderly Study. Am J Clin Nutr. 2007; 85:1142–1147. PMID: 17413117.

78. Kalmijn S, Launer LJ, Ott A, Witteman JC, Hofman A, Breteler MM. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann Neurol. 1997; 42:776–782. PMID: 9392577.

79. Engelhart MJ, Geerlings MI, Ruitenberg A, Van Swieten JC, Hofman A, Witteman JC, Breteler MM. Diet and risk of dementia: Does fat matter?: the Rotterdam Study. Neurology. 2002; 59:1915–1921. PMID: 12499483.

80. Pottala JV, Yaffe K, Robinson JG, Espeland MA, Wallace R, Harris WS. Higher RBC EPA + DHA corresponds with larger total brain and hippocampal volumes: WHIMS-MRI study. Neurology. 2014; 82:435–442. PMID: 24453077.

81. Tan ZS, Harris WS, Beiser AS, Au R, Himali JJ, Debette S, Pikula A, Decarli C, Wolf PA, Vasan RS, Robins SJ, Seshadri S. Red blood cell ω-3 fatty acid levels and markers of accelerated brain aging. Neurology. 2012; 78:658–664. PMID: 22371413.
82. Zhang Y, Chen J, Qiu J, Li Y, Wang J, Jiao J. Intakes of fish and polyunsaturated fatty acids and mild-to-severe cognitive impairment risks: a dose-response meta-analysis of 21 cohort studies. Am J Clin Nutr. 2016; 103:330–340. PMID: 26718417.
83. Dangour AD, Allen E, Elbourne D, Fasey N, Fletcher AE, Hardy P, Holder GE, Knight R, Letley L, Richards M, Uauy R. Effect of 2-y n-3 long-chain polyunsaturated fatty acid supplementation on cognitive function in older people: a randomized, double-blind, controlled trial. Am J Clin Nutr. 2010; 91:1725–1732. PMID: 20410089.

84. Quinn JF, Raman R, Thomas RG, Yurko-Mauro K, Nelson EB, Van Dyck C, Galvin JE, Emond J, Jack CR Jr, Weiner M, Shinto L, Aisen PS. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. JAMA. 2010; 304:1903–1911. PMID: 21045096.
85. Freund-Levi Y, Eriksdotter-Jönhagen M, Cederholm T, Basun H, Faxén-Irving G, Garlind A, Vedin I, Vessby B, Wahlund LO, Palmblad J. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Arch Neurol. 2006; 63:1402–1408. PMID: 17030655.
86. Lee LK, Shahar S, Chin AV, Yusoff NA. Docosahexaenoic acid-concentrated fish oil supplementation in subjects with mild cognitive impairment (MCI): a 12-month randomised, double-blind, placebo-controlled trial. Psychopharmacology (Berl). 2013; 225:605–612. PMID: 22932777.

87. Jiao J, Li Q, Chu J, Zeng W, Yang M, Zhu S. Effect of n-3 PUFA supplementation on cognitive function throughout the life span from infancy to old age: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2014; 100:1422–1436. PMID: 25411277.

88. Yurko-Mauro K, Alexander DD, Van Elswyk ME. Docosahexaenoic acid and adult memory: a systematic review and meta-analysis. PLoS One. 2015; 10:e0120391. PMID: 25786262.

89. Mazereeuw G, Lanctôt KL, Chau SA, Swardfager W, Herrmann N. Effects of ω-3 fatty acids on cognitive performance: a meta-analysis. Neurobiol Aging. 2012; 33:1482.e17–1482.e29.
90. Avallone R, Vitale G, Bertolotti M. Omega-3 fatty acids and neurodegenerative diseases: new evidence in clinical trials. Int J Mol Sci. 2019; 20:4256.

92. Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet. 1997; 349:1436–1442. PMID: 9164317.

93. Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, Leucht S, Ruhe HG, Turner EH, Higgins JP, Egger M, Takeshima N, Hayasaka Y, Imai H, Shinohara K, Tajika A, Ioannidis JP, Geddes JR. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018; 391:1357–1366. PMID: 29477251.

94. Hibbeln JR, Nieminen LR, Blasbalg TL, Riggs JA, Lands WE. Healthy intakes of n-3 and n-6 fatty acids: estimations considering worldwide diversity. Am J Clin Nutr. 2006; 83:1483S–1493S. PMID: 16841858.

95. Connor WE. Importance of n-3 fatty acids in health and disease. Am J Clin Nutr. 2000; 71:171S–175S. PMID: 10617967.

96. Su KP. Biological mechanism of antidepressant effect of omega-3 fatty acids: how does fish oil act as a ‘mind-body interface’? Neurosignals. 2009; 17:144–152. PMID: 19190401.

97. Su KP, Shen WW, Huang SY. Effects of polyunsaturated fatty acids on psychiatric disorders. Am J Clin Nutr. 2000; 72:1241. PMID: 11063464.

98. Lin PY, Huang SY, Su KP. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol Psychiatry. 2010; 68:140–147. PMID: 20452573.

100. Nemets B, Stahl Z, Belmaker RH. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am J Psychiatry. 2002; 159:477–479. PMID: 11870016.

101. Peet M, Horrobin DF. A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch Gen Psychiatry. 2002; 59:913–919. PMID: 12365878.

102. Su KP, Huang SY, Chiu CC, Shen WW. Omega-3 fatty acids in major depressive disorder. A preliminary double-blind, placebo-controlled trial. Eur Neuropsychopharmacol. 2003; 13:267–271. PMID: 12888186.
103. Jazayeri S, Tehrani-Doost M, Keshavarz SA, Hosseini M, Djazayery A, Amini H, Jalali M, Peet M. Comparison of therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. Aust N Z J Psychiatry. 2008; 42:192–198. PMID: 18247193.

104. Su KP, Huang SY, Chiu TH, Huang KC, Huang CL, Chang HC, Pariante CM. Omega-3 fatty acids for major depressive disorder during pregnancy: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2008; 69:644–651. PMID: 18370571.
105. Frangou S, Lewis M, McCrone P. Efficacy of ethyl-eicosapentaenoic acid in bipolar depression: randomised double-blind placebo-controlled study. Br J Psychiatry. 2006; 188:46–50. PMID: 16388069.
106. da Silva TM, Munhoz RP, Alvarez C, Naliwaiko K, Kiss A, Andreatini R, Ferraz AC. Depression in Parkinson's disease: a double-blind, randomized, placebo-controlled pilot study of omega-3 fatty-acid supplementation. J Affect Disord. 2008; 111:351–359. PMID: 18485485.

107. Mischoulon D, Papakostas GI, Dording CM, Farabaugh AH, Sonawalla SB, Agoston AM, Smith J, Beaumont EC, Dahan LE, Alpert JE, Nierenberg AA, Fava M. A double-blind, randomized controlled trial of ethyl-eicosapentaenoate for major depressive disorder. J Clin Psychiatry. 2009; 70:1636–1644. PMID: 19709502.

108. Rapaport MH, Nierenberg AA, Schettler PJ, Kinkead B, Cardoos A, Walker R, Mischoulon D. Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder: a proof-of-concept study. Mol Psychiatry. 2016; 21:71–79. PMID: 25802980.

109. Lespérance F, Frasure-Smith N, St-André E, Turecki G, Lespérance P, Wisniewski SR. The efficacy of omega-3 supplementation for major depression: a randomized controlled trial. J Clin Psychiatry. 2011; 72:1054–1062. PMID: 20584525.
110. Gertsik L, Poland RE, Bresee C, Rapaport MH. Omega-3 fatty acid augmentation of citalopram treatment for patients with major depressive disorder. J Clin Psychopharmacol. 2012; 32:61–64. PMID: 22198441.

111. Martins JG. EPA but not DHA appears to be responsible for the efficacy of omega-3 long chain polyunsaturated fatty acid supplementation in depression: evidence from a meta-analysis of randomized controlled trials. J Am Coll Nutr. 2009; 28:525–542. PMID: 20439549.

112. Bloch MH, Hannestad J. Omega-3 fatty acids for the treatment of depression: systematic review and meta-analysis. Mol Psychiatry. 2012; 17:1272–1282. PMID: 21931319.

113. Martins JG, Bentsen H, Puri BK. Eicosapentaenoic acid appears to be the key omega-3 fatty acid component associated with efficacy in major depressive disorder: a critique of Bloch and Hannestad and updated meta-analysis. Mol Psychiatry. 2012; 17:1144–1149. PMID: 22488258.

114. Grosso G, Pajak A, Marventano S, Castellano S, Galvano F, Bucolo C, Drago F, Caraci F. Role of omega-3 fatty acids in the treatment of depressive disorders: a comprehensive meta-analysis of randomized clinical trials. PLoS One. 2014; 9:e96905. PMID: 24805797.

115. Mocking RJ, Harmsen I, Assies J, Koeter MW, Ruhé HG, Schene AH. Meta-analysis and meta-regression of omega-3 polyunsaturated fatty acid supplementation for major depressive disorder. Transl Psychiatry. 2016; 6:e756. PMID: 26978738.

116. Bai ZG, Bo A, Wu SJ, Gai QY, Chi I. Omega-3 polyunsaturated fatty acids and reduction of depressive symptoms in older adults: a systematic review and meta-analysis. J Affect Disord. 2018; 241:241–248. PMID: 30138808.

117. Hallahan B, Ryan T, Hibbeln JR, Murray IT, Glynn S, Ramsden CE, SanGiovanni JP, Davis JM. Efficacy of omega-3 highly unsaturated fatty acids in the treatment of depression. Br J Psychiatry. 2016; 209:192–201. PMID: 27103682.

118. Lin PY, Su KP. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J Clin Psychiatry. 2007; 68:1056–1061. PMID: 17685742.

119. Lin PY, Mischoulon D, Freeman MP, Matsuoka Y, Hibbeln J, Belmaker RH, Su KP. Are omega-3 fatty acids antidepressants or just mood-improving agents? The effect depends upon diagnosis, supplement preparation, and severity of depression. Mol Psychiatry. 2012; 17:1161–1163. PMID: 22824812.

120. Appleton KM, Sallis HM, Perry R, Ness AR, Churchill R. Omega-3 fatty acids for depression in adults. Cochrane Database Syst Rev. 2015; 2015:CD004692.

121. Su KP, Wang SM, Pae CU. Omega-3 polyunsaturated fatty acids for major depressive disorder. Expert Opin Investig Drugs. 2013; 22:1519–1534.

122. Su KP. Personalized medicine with Omega-3 fatty acids for depression in children and pregnant women and depression associated with inflammation. J Clin Psychiatry. 2015; 76:e1476–7. PMID: 26646045.

123. Chang CH, Tseng PT, Chen NY, Lin PC, Lin PY, Chang JP, Kuo FY, Lin J, Wu MC, Su KP. Safety and tolerability of prescription omega-3 fatty acids: a systematic review and meta-analysis of randomized controlled trials. Prostaglandins Leukot Essent Fatty Acids. 2018; 129:1–12. PMID: 29482765.

124. Su KP. Nutrition, psychoneuroimmunology and depression: the therapeutic implications of omega-3 fatty acids in interferon-α-induced depression. Biomedicine (Taipei). 2015; 5:21. PMID: 26615538.

125. Song C, Shieh CH, Wu YS, Kalueff A, Gaikwad S, Su KP. The role of omega-3 polyunsaturated fatty acids eicosapentaenoic and docosahexaenoic acids in the treatment of major depression and Alzheimer's disease: acting separately or synergistically? Prog Lipid Res. 2016; 62:41–54. PMID: 26763196.

126. Li F, Liu X, Zhang D. Fish consumption and risk of depression: a meta-analysis. J Epidemiol Community Health. 2016; 70:299–304. PMID: 26359502.

127. Liao Y, Xie B, Zhang H, He Q, Guo L, Subramaniapillai M, Fan B, Lu C, Mclntyer RS. Efficacy of omega-3 PUFAs in depression: a meta-analysis. Transl Psychiatry. 2019; 9:190. PMID: 31383846.

128. Guu TW, Mischoulon D, Sarris J, Hibbeln J, McNamara RK, Hamazaki K, Freeman MP, Maes M, Matsuoka YJ, Belmaker RH, Jacka F, Pariante C, Berk M, Marx W, Su KP. International Society for Nutritional Psychiatry Research Practice Guidelines for omega-3 fatty acids in the treatment of major depressive disorder. Psychother Psychosom. 2019; 88:263–273. PMID: 31480057.

129. Larqué E, Gil-Sánchez A, Prieto-Sánchez MT, Koletzko B. Omega 3 fatty acids, gestation and pregnancy outcomes. Br J Nutr. 2012; 107(Suppl 2):S77–S84. PMID: 22591905.

130. Makrides M, Gibson RA, McPhee AJ, Yelland L, Quinlivan J, Ryan P, DOMInO Investigative Team . Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children: a randomized controlled trial. JAMA. 2010; 304:1675–1683. PMID: 20959577.

131. Carlson SE, Colombo J, Gajewski BJ, Gustafson KM, Mundy D, Yeast J, Georgieff MK, Markley LA, Kerling EH, Shaddy DJ. DHA supplementation and pregnancy outcomes. Am J Clin Nutr. 2013; 97:808–815. PMID: 23426033.

132. Shireman TI, Kerling EH, Gajewski BJ, Colombo J, Carlson SE. Docosahexaenoic acid supplementation (DHA) and the return on investment for pregnancy outcomes. Prostaglandins Leukot Essent Fatty Acids. 2016; 111:8–10. PMID: 27499448.

133. de Seymour JV, Simmonds LA, Gould J, Makrides M, Middleton P. Omega-3 fatty acids to prevent preterm birth: Australian pregnant women's preterm birth awareness and intentions to increase omega-3 fatty acid intake. Nutr J. 2019; 18:74. PMID: 31727060.

134. Koletzko B, Poindexter B, Uauy R. Recommended nutrient intake levels for stable, fully enterally fed very low birth weight infants. World Rev Nutr Diet. 2014; 110:297–299. PMID: 24751638.

Go to : 




 PDF
PDF Citation
Citation Print
Print



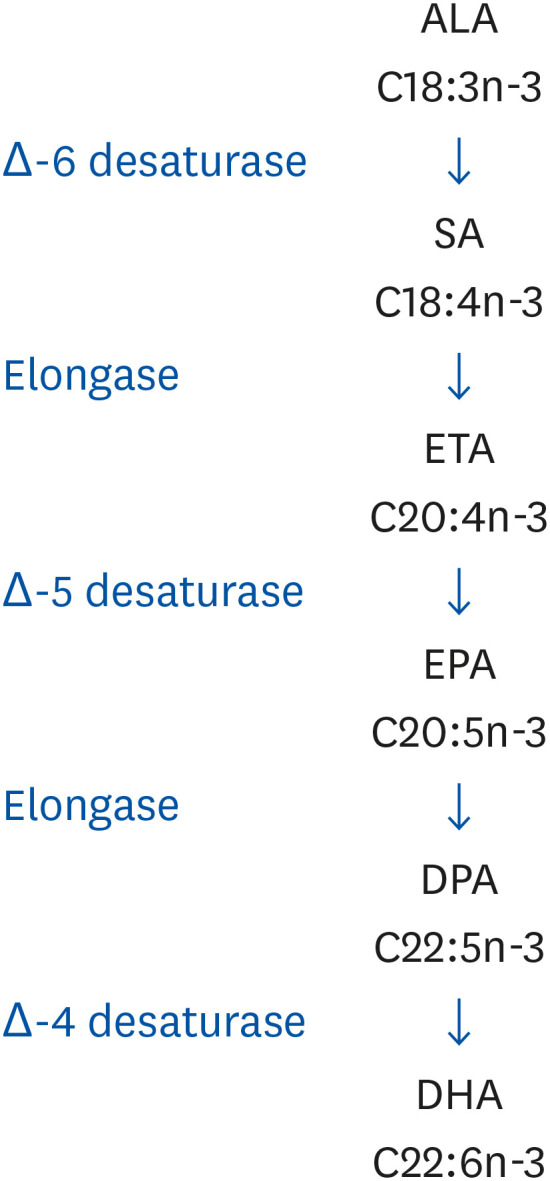
 XML Download
XML Download