1. Vieau D. Perinatal nutritional programming of health and metabolic adult disease. World J Diabetes. 2011; 2:133–136. PMID:
21954417.

2. Kruse M, Seki Y, Vuguin PM, Du XQ, Fiallo A, Glenn AS, Singer S, Breuhahn K, Katz EB, Charron MJ. High-fat intake during pregnancy and lactation exacerbates high-fat diet-induced complications in male offspring in mice. Endocrinology. 2013; 154:3565–3576. PMID:
23861375.

3. Yamazaki M, Yamada H, Munetsuna E, Ishikawa H, Mizuno G, Mukuda T, Mouri A, Nabeshima T, Saito K, Suzuki K, Hashimoto S, Ohashi K. Excess maternal fructose consumption impairs hippocampal function in offspring via epigenetic modification of BDNF promoter. FASEB J. 2018; 32:2549–2562. PMID:
29401579.
4. Bleich SN, Vercammen KA, Koma JW, Li Z. Trends in beverage consumption among children and adults, 2003–2014. Obesity (Silver Spring). 2018; 26:432–441. PMID:
29134763.

5. Sundborn G, Thornley S, Merriman TR, Lang B, King C, Lanaspa MA, Johnson RJ. Are liquid sugars different from solid sugar in their ability to cause metabolic syndrome? Obesity (Silver Spring). 2019; 27:879–887. PMID:
31054268.

6. Lindqvist A, Baelemans A, Erlanson-Albertsson C. Effects of sucrose, glucose and fructose on peripheral and central appetite signals. Regul Pept. 2008; 150:26–32. PMID:
18627777.

7. Hannou SA, Haslam DE, McKeown NM, Herman MA. Fructose metabolism and metabolic disease. J Clin Invest. 2018; 128:545–555. PMID:
29388924.

8. Gaston SA, Tulve NS, Ferguson TF. Abdominal obesity, metabolic dysfunction, and metabolic syndrome in U.S. adolescents: National Health and Nutrition Examination Survey 2011–2016. Ann Epidemiol. 2019; 30:30–36. PMID:
30545765.

9. Li Y, Zhao L, Yu D, Wang Z, Ding G. Metabolic syndrome prevalence and its risk factors among adults in China: a nationally representative cross-sectional study. PLoS One. 2018; 13:e0199293. PMID:
29920555.

10. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr, Spertus JA, Costa F. American Heart Association. National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005; 112:2735–2752. PMID:
16157765.
11. Mulè G, Calcaterra I, Nardi E, Cerasola G, Cottone S. Metabolic syndrome in hypertensive patients: an unholy alliance. World J Cardiol. 2014; 6:890–907. PMID:
25276291.

12. Wynne BM, Mistry AC, Al-Khalili O, Mallick R, Theilig F, Eaton DC, Hoover RS. Aldosterone modulates the association between NCC and ENaC. Sci Rep. 2017; 7:4149. PMID:
28646163.

13. McDonough AA, Nguyen MT. Maintaining balance under pressure: integrated regulation of renal transporters during hypertension. Hypertension. 2015; 66:450–455. PMID:
26101347.
14. Gligorovska L, Bursać B, Kovačević S, Veličković N, Matić G, Djordjevic A. Mif deficiency promotes adiposity in fructose-fed mice. J Endocrinol. 2019; 240:133–145. PMID:
30400058.

15. Wang Y, Thatcher SE, Cassis LA. Measuring blood pressure using a noninvasive tail cuff method in mice. Methods Mol Biol. 2017; 1614:69–73. PMID:
28500596.

16. Seong HY, Cho HM, Kim M, Kim I. Maternal high-fructose intake induces multigenerational activation of the renin-angiotensin-aldosterone system. Hypertension. 2019; 74:518–525. PMID:
31327271.

17. Wu G, Bazer FW, Cudd TA, Meininger CJ, Spencer TE. Maternal nutrition and fetal development. J Nutr. 2004; 134:2169–2172. PMID:
15333699.

18. Chapman DJ, Nommsen-Rivers L. Impact of maternal nutritional status on human milk quality and infant outcomes: an update on key nutrients. Adv Nutr. 2012; 3:351–352. PMID:
22585911.

19. Vickers MH, Clayton ZE, Yap C, Sloboda DM. Maternal fructose intake during pregnancy and lactation alters placental growth and leads to sex-specific changes in fetal and neonatal endocrine function. Endocrinology. 2011; 152:1378–1387. PMID:
21303952.

20. Clayton ZE, Vickers MH, Bernal A, Yap C, Sloboda DM. Early life exposure to fructose alters maternal, fetal and neonatal hepatic gene expression and leads to sex-dependent changes in lipid metabolism in rat offspring. PLoS One. 2015; 10:e0141962. PMID:
26562417.

21. Pereira RM, Botezelli JD, da Cruz Rodrigues KC, Mekary RA, Cintra DE, Pauli JR, da Silva AS, Ropelle ER, de Moura LP. Fructose consumption in the development of obesity and the effects of different protocols of physical exercise on the hepatic metabolism. Nutrients. 2017; 9:405.

22. Softic S, Gupta MK, Wang GX, Fujisaka S, O'Neill BT, Rao TN, Willoughby J, Harbison C, Fitzgerald K, Ilkayeva O, Newgard CB, Cohen DE, Kahn CR. Divergent effects of glucose and fructose on hepatic lipogenesis and insulin signaling. J Clin Invest. 2017; 127:4059–4074. PMID:
28972537.

23. Horton JD, Shimomura I. Sterol regulatory element-binding proteins: activators of cholesterol and fatty acid biosynthesis. Curr Opin Lipidol. 1999; 10:143–150. PMID:
10327282.
24. Kim M, Lee HA, Cho HM, Kang SH, Lee E, Kim IK. Histone deacetylase inhibition attenuates hepatic steatosis in rats with experimental Cushing's syndrome. Korean J Physiol Pharmacol. 2018; 22:23–33. PMID:
29302209.

25. Wakil SJ, Abu-Elheiga LA. Fatty acid metabolism: target for metabolic syndrome. J Lipid Res. 2009; 50(Suppl):S138–43. PMID:
19047759.

26. Mashima T, Seimiya H, Tsuruo T.
De novo fatty-acid synthesis and related pathways as molecular targets for cancer therapy. Br J Cancer. 2009; 100:1369–1372. PMID:
19352381.
27. Nagai Y, Yonemitsu S, Erion DM, Iwasaki T, Stark R, Weismann D, Dong J, Zhang D, Jurczak MJ, Löffler MG, Cresswell J, Yu XX, Murray SF, Bhanot S, Monia BP, Bogan JS, Samuel V, Shulman GI. The role of peroxisome proliferator-activated receptor gamma coactivator-1 beta in the pathogenesis of fructose-induced insulin resistance. Cell Metab. 2009; 9:252–264. PMID:
19254570.
28. Tomlinson JW, Finney J, Gay C, Hughes BA, Hughes SV, Stewart PM. Impaired glucose tolerance and insulin resistance are associated with increased adipose 11beta-hydroxysteroid dehydrogenase type 1 expression and elevated hepatic 5alpha-reductase activity. Diabetes. 2008; 57:2652–2660. PMID:
18633104.
29. DiStefano JK. Fructose-mediated effects on gene expression and epigenetic mechanisms associated with NAFLD pathogenesis. Cell Mol Life Sci. 2020; 77:2079–2090. PMID:
31760464.

30. Cho HM, Kim I. Maternal high-fructose intake induces hypertension through activating histone codes on the (pro)renin receptor promoter. Biochem Biophys Res Commun. 2020; 527:596–602. PMID:
32423811.

31. Horita S, Nakamura M, Suzuki M, Satoh N, Suzuki A, Homma Y, Nangaku M. The role of renal proximal tubule transport in the regulation of blood pressure. Kidney Res Clin Pract. 2017; 36:12–21. PMID:
28428931.

32. Zhuo JL, Li XC. Proximal nephron. Compr Physiol. 2013; 3:1079–1123. PMID:
23897681.

33. Cogan MG. Angiotensin II: a powerful controller of sodium transport in the early proximal tubule. Hypertension. 1990; 15:451–458. PMID:
2185149.

34. Prasad GV. Metabolic syndrome and chronic kidney disease: current status and future directions. World J Nephrol. 2014; 3:210–219. PMID:
25374814.

35. Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014; 24:R453–62. PMID:
24845678.

36. Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007; 87:245–313. PMID:
17237347.

37. Stasia MJ. CYBA encoding p22(phox), the cytochrome b558 alpha polypeptide: gene structure, expression, role and physiopathology. Gene. 2016; 586:27–35. PMID:
27048830.

38. Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal. 2011; 15:1583–1606. PMID:
21473702.

39. Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006; 444:860–867. PMID:
17167474.

40. Catrysse L, van Loo G. Inflammation and the metabolic syndrome: the tissue-specific functions of NF-κB. Trends Cell Biol. 2017; 27:417–429. PMID:
28237661.

41. DiNicolantonio JJ, Mehta V, Onkaramurthy N, O'Keefe JH. Fructose-induced inflammation and increased cortisol: a new mechanism for how sugar induces visceral adiposity. Prog Cardiovasc Dis. 2018; 61:3–9. PMID:
29225114.

42. Kawasaki N, Asada R, Saito A, Kanemoto S, Imaizumi K. Obesity-induced endoplasmic reticulum stress causes chronic inflammation in adipose tissue. Sci Rep. 2012; 2:799. PMID:
23150771.

43. Song D, Arikawa E, Galipeau D, Battell M, McNeill JH. Androgens are necessary for the development of fructose-induced hypertension. Hypertension. 2004; 43:667–672. PMID:
14757778.

44. Vasudevan H, Xiang H, McNeill JH. Differential regulation of insulin resistance and hypertension by sex hormones in fructose-fed male rats. Am J Physiol Heart Circ Physiol. 2005; 289:H1335–42. PMID:
15951347.

45. Vasudevan H, Yuen VG, McNeill JH. Testosterone-dependent increase in blood pressure is mediated by elevated Cyp4A expression in fructose-fed rats. Mol Cell Biochem. 2012; 359:409–418. PMID:
21894443.

46. Sharma N, Li L, Ecelbarger CM. Sex differences in renal and metabolic responses to a high-fructose diet in mice. Am J Physiol Renal Physiol. 2015; 308:F400–10. PMID:
25537743.

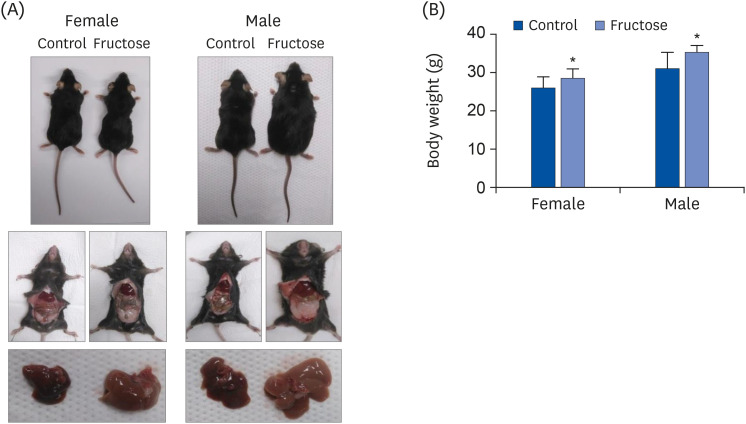
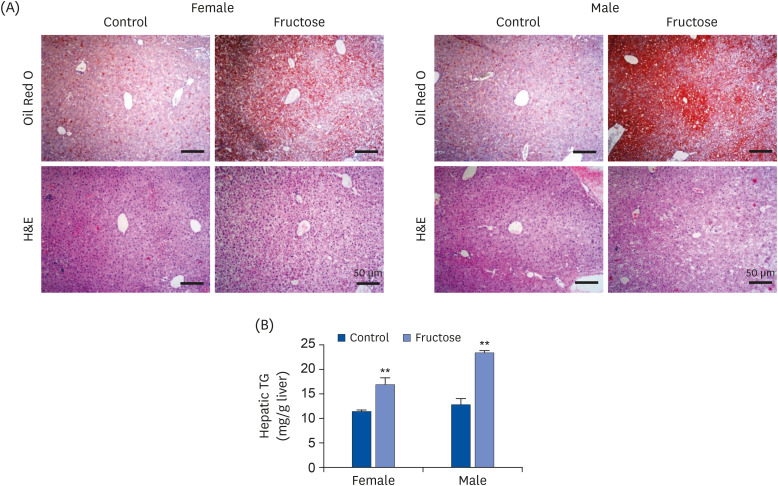
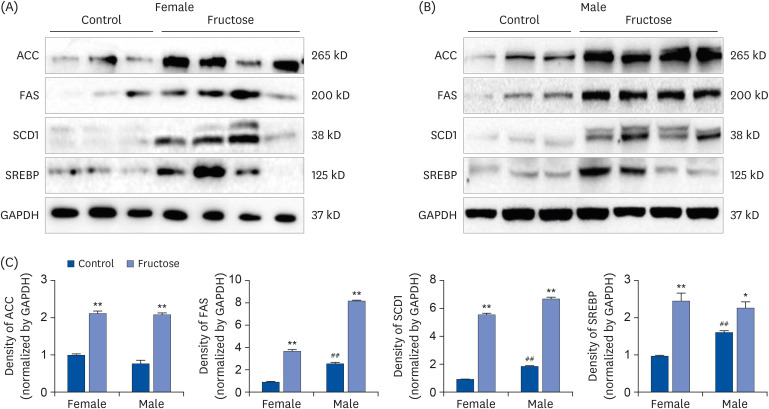
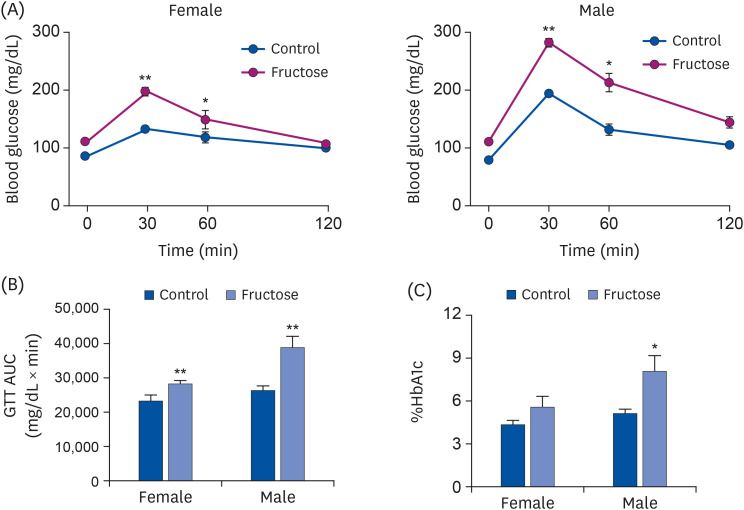




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download