Abstract
Background
We investigated whether distancemax, that is, the degree of distance between the upper aerodigestive tract (UAT) mass and the farthest pathologic lymph node, was significantly associated with survival in patients with limited-stage UAT natural killer/T cell lymphoma (NKTCL).
Methods
A total of 157 patients who received chemotherapy (CTx) with/without radiotherapy (RTx) were enrolled.
Results
In the survival analysis, an elevated lactate dehydrogenase level [progression-free survival (PFS) hazard ratio (HR), 2.948; 95% confidence interval (CI), 1.606‒5.404; P<0.001; overall survival (OS) HR, 2.619; 95% CI, 1.594‒4.822; P=0.003], short distancemax (PFS HR, 0.170; 95% CI, 0.071‒0.410; P<0.001; OS HR, 0.142; 95% CI, 0.050‒0.402; P< 0.001), and CTx combined with RTx (HR, 0.168; 95%CI, 0.079‒0.380; P<0.001; OS HR, 0.193; 95% CI, 0.087‒0.429; P<0.001) had an independent predictive value for PFS and OS.
Extranodal (EN) nasal-type natural killer/T-cell lymphoma (NKTCL) is a rare but aggressive T-cell lymphoma [1-3]. The epidemiology of this disease has significant geographical variation, with a higher incidence in East Asian and Latin American populations [4].
An EN NKTCL can be further classified according to the anatomic sites of the primary mass: a nasal type NKTCL, involving the upper aerodigestive tract (UAT) and an extra-nasal NKTCL, involving the sites outside the UAT [5]. The UAT NKTCL has well-defined morphological, genetic, and clinical characteristics. It has several clinical features such as angioinvasion, tumor necrosis, and frequent association with Epstein-Barr virus, predilection for young males, and more importantly, early-stage detection of most nasal types of NKTCLs [6-8].
Approximately 80% of NKTCLs are localized. In recent years, it has attracted increasing attention, and consensus on treatment has gradually been established. However, there is no standard staging system to date. The Ann Arbor (AA) staging system, which was originally designed for Hodgkin lymphoma, is conventionally used for NKTCLs. However, this staging method has limited utility in prognostication and treatment decision-making in patients with NKTCL, as the NKTCL is almost exclusively EN and the majority is localized at diagnosis. Thus, the AA staging system has a fatal problem for staging localized UAT NKTCLs. Moreover, the staging of the lymphatic spread in the AA staging system is divided depending on the diaphragm; thus, it is not valuable for localized NKTCLs.
The prognostic impact of the International Prognostic Index (IPI) has been controversial for UAT NKTCLs due to heterogeneous data from several studies [9-12]. In particular, a small population of patients with UAT NKTCL is only included in the high IPI score group [10-14]. Therefore, it might be an inappropriate prognostic system for localized UAT NKTCLs.
Kim et al. [15] classified the nasal type UAT NKTCL into limited disease [AA stage I/II without local tumor invasiveness (LTI)] and extensive disease (stage I/II with LTI and stage III/IV). However, recent data have demonstrated that regional lymph node (LN) involvement in the limited stage is significantly associated with poor prognosis in UAT NKTCLs [16]. Moreover, a single data set proposed the tumor, node, and metastasis (TNM) stage in nasal NKTCLs, likely head and neck cancer [17]. In the data, the authors classified patients into no, unilateral, and bilateral LN involvement, as the prognosis in the three groups was significantly different. Thus, no, unilateral, and bilateral involvement was defined as N0, N1, and N2 stage, respectively in the TNM stage of nasal NKTCL.
However, a recent multicenter study showed that the overall survival (OS) did not differ significantly between patients with and without regional LN involvement because of improved local control due to the early use of concurrent chemoradiotherapy [18]. Thus, we hypothesized that the degree of lymphatic spread might have a prognostic significance in limited-stage UAT NKTCLs because advanced spreading of LN involvement could make proper control and treatment of the disease more difficult.
In the present study, we investigated the clinical impact of the degree of the distance between the UAT EN mass and the farthest pathologic LN. Based on a comparative analysis with other available prognostic factors, it is important to predict survival in patients with limited-stage UAT NKTCL.
Between January 2008 and October 2017, 157 patients with AA stage IE and IIE UAT NKTCLs were enrolled at five medical centers: Hanyang University Hanmaeum Changwon Hospital, Pusan National University Hospital, Dong-A University Hospital, Busan Paik Hospital, and Busan Haeundae Paik Hospital. Approval for the retrospective review of electronic medical records was obtained from the Institutional Review Boards of the medical centers.
Diagnosis of an EN NKTCL was made based on the presence of relevant histologic findings and immunophenotypes, such as CD3+, CD20-, and CD56+ with or without positive Epstein-Barr virus by fluorescence in situ hybridization. Moreover, the patients were required to be in a limited stage (AA stage I and II without LTI) and previously untreated with no previous concomitant malignancies, and the primary tumor was required to be located in the UAT. Patients with active pulmonary tuberculosis, viral hepatitis, rheumatoid arthritis, and chronic renal disease were excluded. Tumor response was assessed using the standard response criteria [19].
Immunohistochemistry studies were performed on 3-mm-thick sections obtained from formalin-fixed, paraffin-embedded samples. Histological staining with Ki-67 (1:100 clone 7B11, Zymed Lab Inc., San Francisco, CA, USA) antibodies was performed using the standard avidin-biotin-peroxidase complex method as described elsewhere. Heat-induced antigen retrieval was performed for 45 min in a Tris–ethylenediaminetetraacetic acid buffer at pH 8 for the antibody in a pressure cooker at 95°C. The cut-off value for Ki-67 expression was 70%. High expression was defined as a Ki-67 expression level ≥70%.
Dual-modality 18F-FDG PET/CT was performed using a Biograph instrument (Siemens Medical Solutions, Hoffman Estates, IL, USA) based on dual-slice helical CT and full-ring PET tomography.
PET images were evaluated to detect areas with increased FDG tracer uptake. The lymphoma area with an increased FDG tracer uptake [standard uptake value (SUV) ≥2.5] in a contouring border, and a pathologic lesion diameter ≥1.0 cm was considered as an active lymphoma lesion. In the PET/CT imaging sections, CT scans were used for PET attenuation correction.
Distancemax was defined as the linear distance between the main UAT mass and the farthest pathologic LN in sagittal, coronal, and transverse sections of the initial PET/CT scan; thus, we measured the length of the distancemax. Distancemax in stage I was defined as zero because we did not measure the distance. Furthermore, SUVmax was defined as the highest SUV among the maximum value of SUV in each tumorous area or LN measured by PET/CT scan. The SUVmax values were also measured in the initial PET/CT.
A chi-square test or Fisher’s exact test was used as appropriate to analyze categorical variables. A Cox proportional hazard regression model was used to identify independent risk factors for survival in patients with limited-stage UAT ENKTCL.
Progression-free survival (PFS) was calculated from the date of diagnosis to the date of progression, relapse, or the last follow-up date. The OS was measured from the date of diagnosis to the date of death due to the tumor or the last follow-up date. The Kaplan-Meier method was used to derive the PFS and OS curves. Differences in the survival curves were calculated using the log-rank test.
Receiver operating characteristic (ROC) curves were prepared to estimate the measurement accuracy of the optimal cut-off values of continuous variables such as distancemax and SUVmax. Statistical analysis was performed using SPSS software (version 18.0; SPSS Inc., Chicago, IL, USA). Statistical significance was set at P<0.05.
The baseline characteristics of the 157 patients with limited-stage UAT ENKTCL are summarized in Table 1. One hundred and eight men (68.8%) and 49 women (31.2%) with a median age of 58 years (range, 29–78 yr) were included in this study. The primary sites of lymphoma were the nasal cavity in 117 patients (74.5%), nasopharynx in 34 (21.6%), and oral cavity/oropharynx in 6 (3.8%). A total of 20 patients (12.7%) were in AA stage I, while 137 patients (87.3%) were in stage II. A total of 31 patients (19.7%) had elevated lactate dehydrogenase levels, and 25 patients (15.9%) had elevated Ki-67 scores above 70%. Furthermore, information about the presence of B symptoms, Eastern Cooperative Oncology Group (ECOG) performance status (PS), bulky disease, bilateral regional LN involvement, therapeutic modality, and SUVmax in PET/CT are also described in Table 1.
Fifty-nine patients (37.6%) received VIPD therapy (etoposide 100 mg/m2 on days 1–3, ifosfamide 1.5 g/m2 on days 1–3, cisplatin 33 mg/m2 on days 1–3, and dexamethasone 40 mg on days 1–4) with/without RTx. Sixty-eight patients (43.3%) received VPDL therapy (etoposide 100 mg/m2 on days 1–3, ifosfamide 1.2 g/m2 on days 1–3, dexamethasone 40 mg IV, and L-asparaginase on days 8, 10, 14, 16, 18, and 20) with/without RTx. Moreover, 16 patients (10.2%) received DeVIC therapy (dexamethasone, etoposide, ifosfamide, and carboplatin) and 14 patients (8.9%) received SMILE therapy (solumedrol, methotrexate, ifosfamide, L-asparaginase, and etoposide).
Combined modality treatment schedules included RTx followed by non-anthracycline CTx or non-anthracycline CTx followed by RTx [median cycle, 3 cycles (range, 3–4 cycles)]. The median dose of RTx was 45.0 Gy (range, 38.0–50.0 Gy).
The ROC analysis was performed to measure the optimal cut-off values of distancemax and SUVmax as continuous variables (Fig. 1). The cut-off values of distancemax and SUVmax were 10.8 cm and 7.1, respectively. According to the cut-off value of distancemax, 90 patients (57.3%) were included in the long distancemax group, and 67 patients (42.7%) were in the short distancemax group. Differences in the PFS and OS between the long and short distancemax groups were significant (PFS, P<0.001, Fig. 2A; OS, P<0.001, Fig. 2B). Furthermore, the high and low SUVmax groups were dichotomized using the above cut-off value of SUVmax. In the analysis, differences in the PFS between the high and low SUVmax groups were also very significant (P<0.001). However, the OS did not differ between the two groups (P<0.056).
We also performed univariate analysis to determine the clinical impact of the other available prognostic factors including male sex (PFS, P=0.059; OS, P=0.458), the presence of B Symptoms (PFS, P=0.568; OS, P=0.504), stage II (PFS, P=0.007; OS, P=0.076), elevated LDH levels (PFS, P<0.001; OS, P=0.009), ECOG PS ≥grade 2 (PFS, P=0.003; OS, P=0.016), high Ki-67 levels ≥70% (PFS, P=0.007; OS, P=0.068), regional LN involvement (PFS, P=0.034; OS, P=0.386), bilateral LN involvement (PFS, P<0.001; OS, P=0.003), bulky disease ≥7.5 cm (PFS, P=0.531; OS, P=0.334), and CTx combined with RTx (PFS, P<0.001; OS, P<0.001).
Multivariate analysis was performed on the significant prognostic factors in the univariate analysis. In the analysis, elevated LDH level [PFS: hazard ratio (HR), 2.948; 95% confidence interval (CI), 1.606–5.404; P<0.001; OS: HR, 2.619; 95% CI, 1.594–4.822; P=0.003)], short distancemax (PFS: HR, 0.170; 95% CI, 0.071–0.410; P<0.001; OS: HR, 0.142; 95% CI, 0.050–0.402; P<0.001) and CTx combined with RTx (HR, 0.168; 95% CI, 0.079–0.380; P<0.001; OS: HR, 0.193; 95% CI, 0.087–0.429; P<0.001) had an independent predictive value for PFS and OS (Table 2).
We compared prognosis according to distancemax as the degree of lymphatic spread and therapeutic modalities such as CTx with/without RTx, since distancemax and CTx combined with RTx were independent prognostic factors for survival in patients with limited-stage UAT NKTCL. In the Kaplan-Meier survival curve, lymphoma involvement within a short distancemax in patients treated with CTx and RTx had the most favorable PFS and OS (PFS, P<0.001; Fig. 3A; OS, P<0.001, Fig. 3B). Meanwhile, the involvement within a long distancemax in patients who received only CTx had worst PFS and OS (PFS, P<0.001; OS, P<0.001).
Lymphoma involvement within a short distancemax in patients treated with only CTx and a long distancemax involvement in patients receiving CTx combined with RTx was an intermediate prognostic group. Differences in survival between the two subgroups were not observed (PFS, P=0.431; OS, P=0.578).
In previous studies, most patients with limited-stage UAT NKTCLs have been categorized as low or low-intermediate risk using the IPI score [10-14]. Thus, a novel risk stratification model to classify them into a more subdivided risk group than the IPI is required. Furthermore, a study demonstrated that the IPI score could not properly predict complete response and disease-free survival in multivariate analysis [15]. The above findings suggest that other risk stratification models besides the IPI score for predicting survival would be strongly needed in limited UAT NKTCLs.
A previous clinical study proposed the Korean Prognostic Index, which incluses B symptoms, stage, elevated LDH level, and regional LN involvement in an anthracycline-based CTx setting. In particular, the data showed that regional LN involvement is a novel independent risk factor for a worse OS [16]. Meanwhile, another previous study demonstrated that 5-year disease-free survival was shortened in advanced TNM stage in IE/IIE stage lymphoma of the nasal cavity and paranasal sinus [20]. Similarly, other data showed that the TNM stage is correlated with the extent of the disease in lymphoma [21]. In addition, a recent study showed that the TNM stage is more effective in stratifying tumor burden and risk of prognosis than the AA stage in localized nasal NKTCL [19]. The above data suggest the clinical significance of the TNM staging system in localized UAT area lymphomas. Although the AA staging system could not classify the risk stratification groups, which includes tumor size, invasion to adjacent important structures, and regional LN involvement in the localized NKTCL, it is not thought to be a useful staging system for localized tumors.
Various studies have demonstrated that regional LN involvement is the most important prognostic factor in localized UAT NKTCLs [1, 14, 16, 22, 23]. However, the prognostic index of natural killer cell lymphoma consisting of four risk factors including age, stage, non-nasal type, and distant LN involvement, which was proposed in a recent study, showed that regional LN involvement itself is not significantly associated with survival in NKTCL [18]. We also found that regional LN involvement itself does not properly predict prognosis from parallel analysis with several other risk factors in patients with limited-stage UAT NKTCL. Thus, we measured distancemax from the primary EN site to the farthest LN and then analyzed the clinical impact of distancemax. In the analysis, it was an independent prognostic factor for predicting survival in our patients.
In particular, in the proposed TNM stage in a study of localized nasal NKTCL, N2 stage with bilateral LN involvement had a poorer prognosis than N1 stage with unilateral involvement [17]. However, our study showed that bilateral LN involvement did not significantly reflect the prognosis in limited-stage UAT NKTCLs. This finding suggests that bilateral LN involvement in the radiation field is susceptible to control by RTx or concurrent chemoradiotherapy. For the same reason, shortly involved pathologic LNs were significantly associated with a favorable prognosis in our patients. In contrast, long-distance LN involvement may be beyond the control field. This result suggests that more advanced LN involvement can increase the opportunity for disease progression. Our study showed that lymphoma involvement within a short distancemax in patients treated with CTx with RTx had the best prognosis. Meanwhile, the involvement within a long distancemax in patients who received only CTx had the worst outcomes.
The present study showed that elevated LDH levels and concurrent chemoradiotherapy were also significant prognostic factors for PFS and OS in patients with limited-stage UAT NKTCLs. Elevated LDH level has been a commonly mentioned significant prognostic factor for predicting survival, and it has also been confirmed as an independent prognostic factor in our data. Moreover, CTx combined with RTx was significantly associated with prognosis. CTx followed by RTx or CTx after RTx showed excellent efficacy of sequential therapies for survival in our patients.
Our study has some limitations, such as its retrospective design, small number of patients, and various CTx regimens. In particular, several CTx regimens may have different short-term efficacies. Generally, some CTx regimens, such as L-asparaginase-containing protocols, could show more favorable short-term efficacy than other therapies. However, there are no clear data showing significant differences in efficacy among several therapeutic regimens. Thus, we did not compare differences in survival according to the CTx regimens.
In the present study, local control by chemo-radiotherapy might also be essential for the treatment of patients. More importantly, we found the clinical significance of distancemax reflecting the degree of lymphatic spread for survival in patients with limited NKTCL. We expect that well-designed clinical studies will confirm our findings.
REFERENCES
1. Au WY, Weisenburger DD, Intragumtornchai T, et al. 2009; Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood. 113:3931–7. DOI: 10.1182/blood-2008-10-185256. PMID: 19029440.

2. Suzuki R, Suzumiya J, Yamaguchi M, et al. 2010; Prognostic factors for mature natural killer (NK) cell neoplasms: aggressive NK cell leukemia and extranodal NK cell lymphoma, nasal type. Ann Oncol. 21:1032–40. DOI: 10.1093/annonc/mdp418. PMID: 19850638.

3. Kim TM, Lee SY, Jeon YK, et al. 2008; Clinical heterogeneity of extranodal NK/T-cell lymphoma, nasal type: a national survey of the Korean Cancer Study Group. Ann Oncol. 19:1477–84. DOI: 10.1093/annonc/mdn147. PMID: 18385201.

4. Chan JY, Lim ST. 2018; Novel findings from the Asian Lymphoma Study Group: focus on T and NK-cell lymphomas. Int J Hematol. 107:413–9. DOI: 10.1007/s12185-018-2406-6. PMID: 29380182.

5. Jo JC, Yoon DH, Kim S, et al. 2012; Clinical features and prognostic model for extranasal NK/T-cell lymphoma. Eur J Haematol. 89:103–10. DOI: 10.1111/j.1600-0609.2012.01796.x. PMID: 22553935.

6. Avilés A, Díaz NR, Neri N, Cleto S, Talavera A. 2000; Angiocentric nasal T/natural killer cell lymphoma: a single centre study of prognostic factors in 108 patients. Clin Lab Haematol. 22:215–20. DOI: 10.1046/j.1365-2257.2000.00307.x. PMID: 11012633.

7. Li CC, Tien HF, Tang JL, et al. 2004; Treatment outcome and pattern of failure in 77 patients with sinonasal natural killer/T-cell or T-cell lymphoma. Cancer. 100:366–75. DOI: 10.1002/cncr.11908. PMID: 14716773.

8. Ko YH, Cho EY, Kim JE, et al. 2004; NK and NK-like T-cell lymphoma in extranasal sites: a comparative clinicopathological study according to site and EBV status. Histopathology. 44:480–9. DOI: 10.1111/j.1365-2559.2004.01867.x. PMID: 15139996.

9. Cheung MM, Chan JK, Lau WH, et al. 1998; Primary non-Hodgkin's lymphoma of the nose and nasopharynx: clinical features, tumor immunophenotype, and treatment outcome in 113 patients. J Clin Oncol. 16:70–7. DOI: 10.1200/JCO.1998.16.1.70. PMID: 9440725.

10. Cheung MM, Chan JK, Lau WH, Ngan RK, Foo WW. 2002; Early stage nasal NK/T-cell lymphoma: clinical outcome, prognostic factors, and the effect of treatment modality. Int J Radiat Oncol Biol Phys. 54:182–90. DOI: 10.1016/S0360-3016(02)02916-4. PMID: 12182990.

11. Chim CS, Ma SY, Au WY, et al. 2004; Primary nasal natural killer cell lymphoma: long-term treatment outcome and relationship with the International Prognostic Index. Blood. 103:216–21. DOI: 10.1182/blood-2003-05-1401. PMID: 12933580.

12. You JY, Chi KH, Yang MH, et al. 2004; Radiation therapy versus chemotherapy as initial treatment for localized nasal natural killer (NK)/T-cell lymphoma: a single institute survey in Taiwan. Ann Oncol. 15:618–25. DOI: 10.1093/annonc/mdh143. PMID: 15033670.
13. Kim K, Kim WS, Jung CW, et al. 2002; Clinical features of peripheral T-cell lymphomas in 78 patients diagnosed according to the Revised European-American lymphoma (REAL) classification. Eur J Cancer. 38:75–81. DOI: 10.1016/S0959-8049(01)00344-6. PMID: 11750843.

14. Lee J, Park YH, Kim WS, et al. 2005; Extranodal nasal type NK/T-cell lymphoma: elucidating clinical prognostic factors for risk-based stratification of therapy. Eur J Cancer. 41:1402–8. DOI: 10.1016/j.ejca.2005.03.010. PMID: 15963893.

15. Kim TM, Park YH, Lee SY, et al. 2005; Local tumor invasiveness is more predictive of survival than International Prognostic Index in stage I(E)/II(E) extranodal NK/T-cell lymphoma, nasal type. Blood. 106:3785–90. DOI: 10.1182/blood-2005-05-2056. PMID: 16109779.

16. Lee J, Suh C, Park YH, et al. 2006; Extranodal natural killer T-cell lymphoma, nasal-type: a prognostic model from a retrospective multicenter study. J Clin Oncol. 24:612–8. DOI: 10.1200/JCO.2005.04.1384. PMID: 16380410.

17. Yan Z, Huang HQ, Wang XX, et al. 2015; A TNM staging system for nasal NK/T-cell lymphoma. PLoS One. 10:e0130984. DOI: 10.1371/journal.pone.0130984. PMID: 26098892. PMCID: PMC4476596.

18. Kim SJ, Yoon DH, Jaccard A, et al. 2016; A prognostic index for natural killer cell lymphoma after non-anthracycline-based treatment: a multicentre, retrospective analysis. Lancet Oncol. 17:389–400. DOI: 10.1016/S1470-2045(15)00533-1. PMID: 26873565.

19. Cheson BD, Horning SJ, Coiffier B, et al. 1999; Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 17:1244. DOI: 10.1200/JCO.1999.17.4.1244. PMID: 10561185.
20. Robbins KT, Fuller LM, Vlasak M, et al. 1985; Primary lymphomas of the nasal cavity and paranasal sinuses. Cancer. 56:814–9. DOI: 10.1002/1097-0142(19850815)56:4<814::AID-CNCR2820560419>3.0.CO;2-P. PMID: 4016673.

21. Logsdon MD, Ha CS, Kavadi VS, Cabanillas F, Hess MA, Cox JD. 1997; Lymphoma of the nasal cavity and paranasal sinuses: improved outcome and altered prognostic factors with combined modality therapy. Cancer. 80:477–88. DOI: 10.1002/(SICI)1097-0142(19970801)80:3<477::AID-CNCR16>3.0.CO;2-U. PMID: 9241082.
22. Li YX, Fang H, Liu QF, et al. 2008; Clinical features and treatment outcome of nasal-type NK/T-cell lymphoma of Waldeyer ring. Blood. 112:3057–64. DOI: 10.1182/blood-2008-05-160176. PMID: 18676879.

23. Li YX, Liu QF, Fang H, et al. 2009; Variable clinical presentations of nasal and Waldeyer ring natural killer/T-cell lymphoma. Clin Cancer Res. 15:2905–12. DOI: 10.1158/1078-0432.CCR-08-2914. PMID: 19318493.

Fig. 1
Receiver operating characteristic (ROC) curves to identify optimal cutoff value of maximum distance (distancemax) from primary extra nodal site to the farthest lymph node, and maximum standard uptake value (SUVmax) in patients with limited stage upper aerodigestive tract natural killer/T cell lymphoma. The calculated optimal cutoffs for distancemax and SUVmax were 10.8 and 7.1 respectively. In addition, the area under the ROC curve (AUC) values for distancemax and SUVmax were 0.874 and 0.632, respectively. The AUC value for distancemax was significantly higher than that for SUVmax (P<0.001).
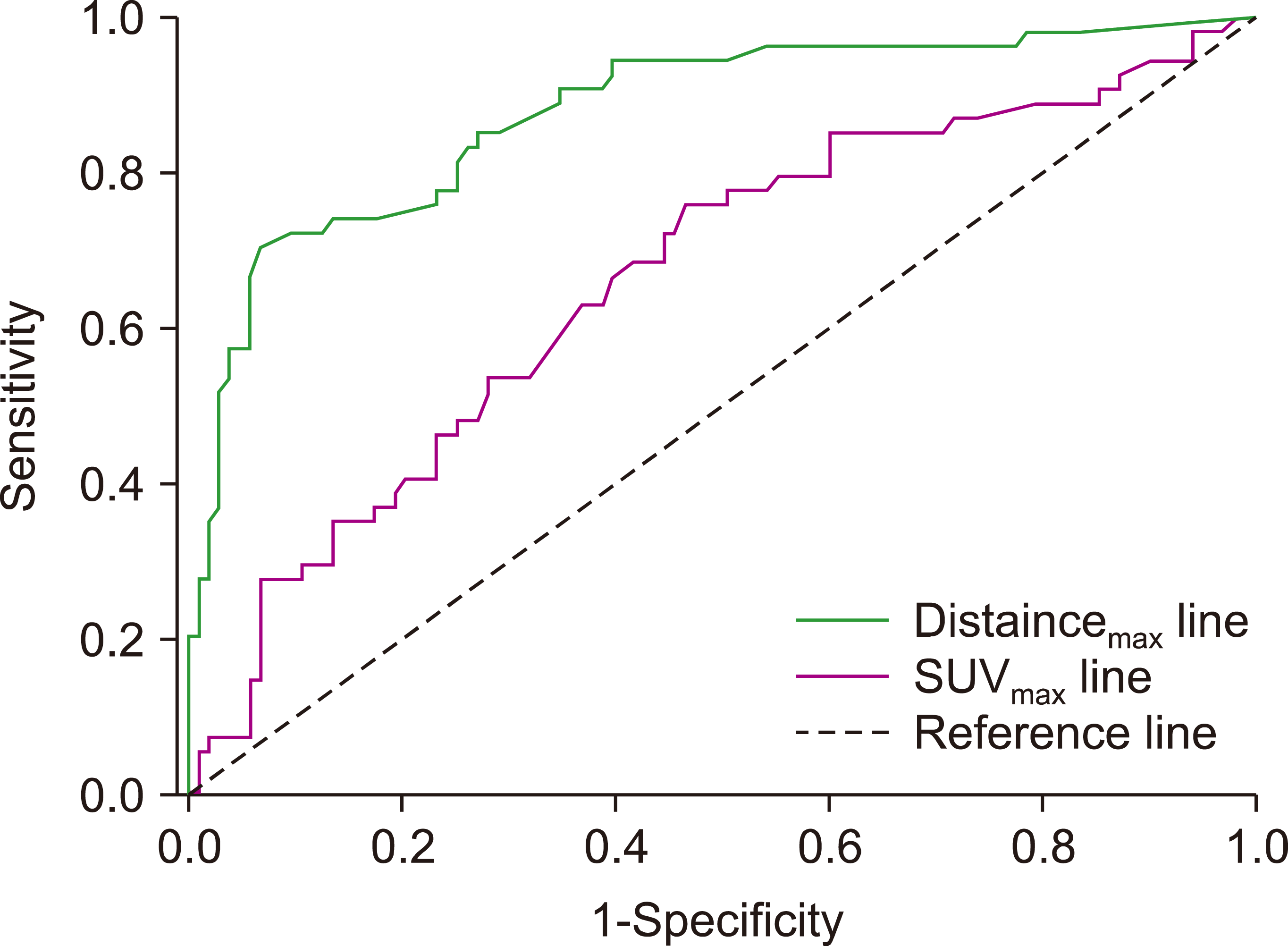
Fig. 2
Survival analysis according to distancemax in patients with limited stage upper aerodigestive tract natural killer/T cell lymphoma. Differences in the short distancemax group (N=67) and long distancemax group (N=90) were significant (progression-free survival, P<0.001; Fig. 2A; OS, P<0.001; Fig. 2B).
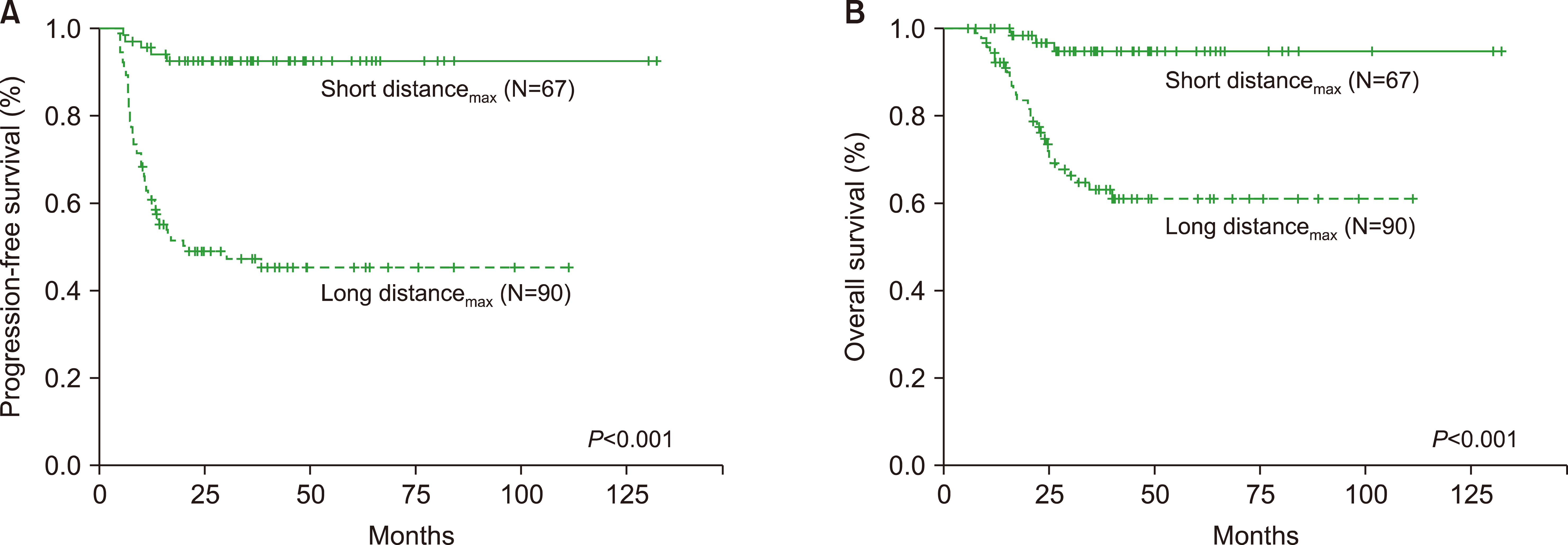
Fig. 3
Prognosis according to degree of lymphatic spread and therapeutic modality. In the Kaplan-Meier survival curve, lymphoma involvement of short distancemax in patients treated with CTx and RTx had the most favorable PFS and OS (PFS, P<0.001; Fig. 3A; OS, P<0.001, Fig. 3B). Meanwhile, involvement of long distancemax in patients received only CTx had worst PFS and OS (PFS, P<0.001, Fig. 3A; OS, P<0.001, Fig. 3B). Lymphoma involvement of short distancemax in patients treated with only CTx and long distancemax involvement in patients who received chemotherapy combined with radiotherapy did not have significant survival differences (PFS, P=0.431; OS, P=0.578).
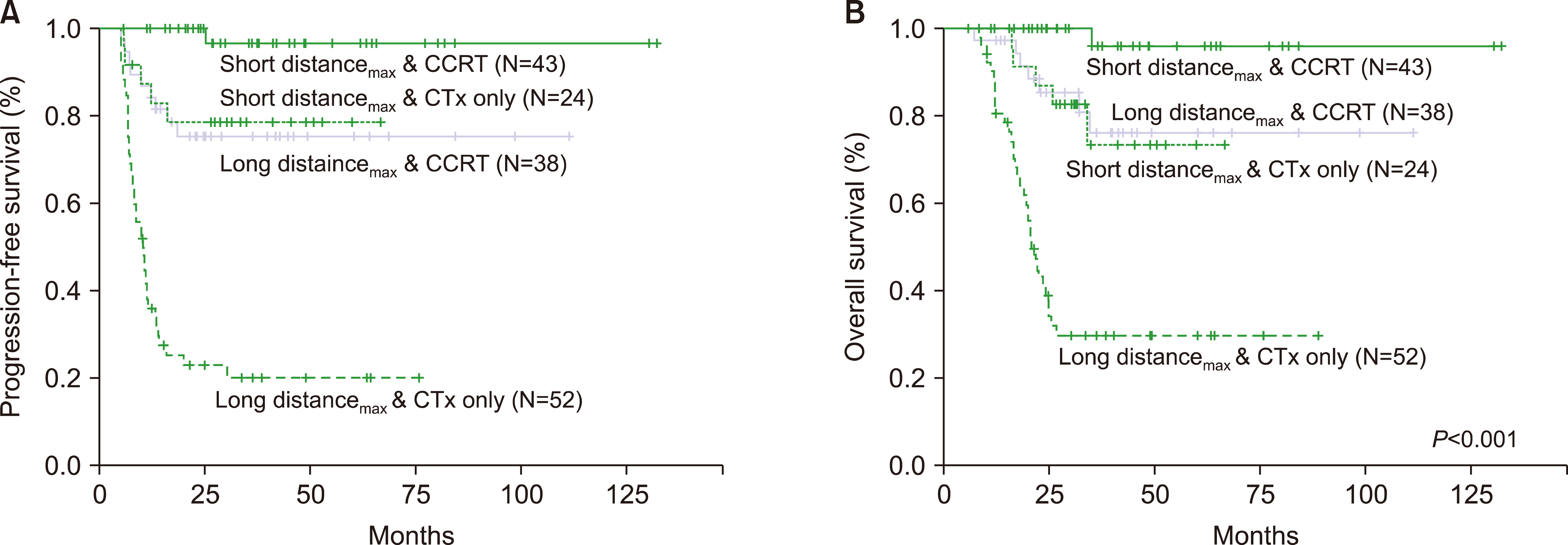
Table 1
Baseline characteristics of patients with limited stage natural killer/T cell lymphoma.
Table 2
Univariate and multivariate analysis of prognostic factors for survival in 157 patients with limited natural killer/T cell lymphoma, nasal type.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download