This article has been
cited by other articles in ScienceCentral.
Abstract
Background
We analyzed cell-free serum Epstein‒Barr virus (EBV) DNA to identify its prognostic role in patients with newly diagnosed lymphoma.
Methods
We retrospectively reviewed patients diagnosed with lymphoma between January 2014 and July 2020. Patients were enrolled according to the following criteria i) pathologically confirmed lymphomas according to the World Health Organization criteria, ii) age over 18 years, iii) serum EBV DNA measurement using polymerase chain reaction prior to first-line therapy, and iv) receipt of curative standard chemotherapy. In total, 263 patients met these criteria and were included in this study.
Results
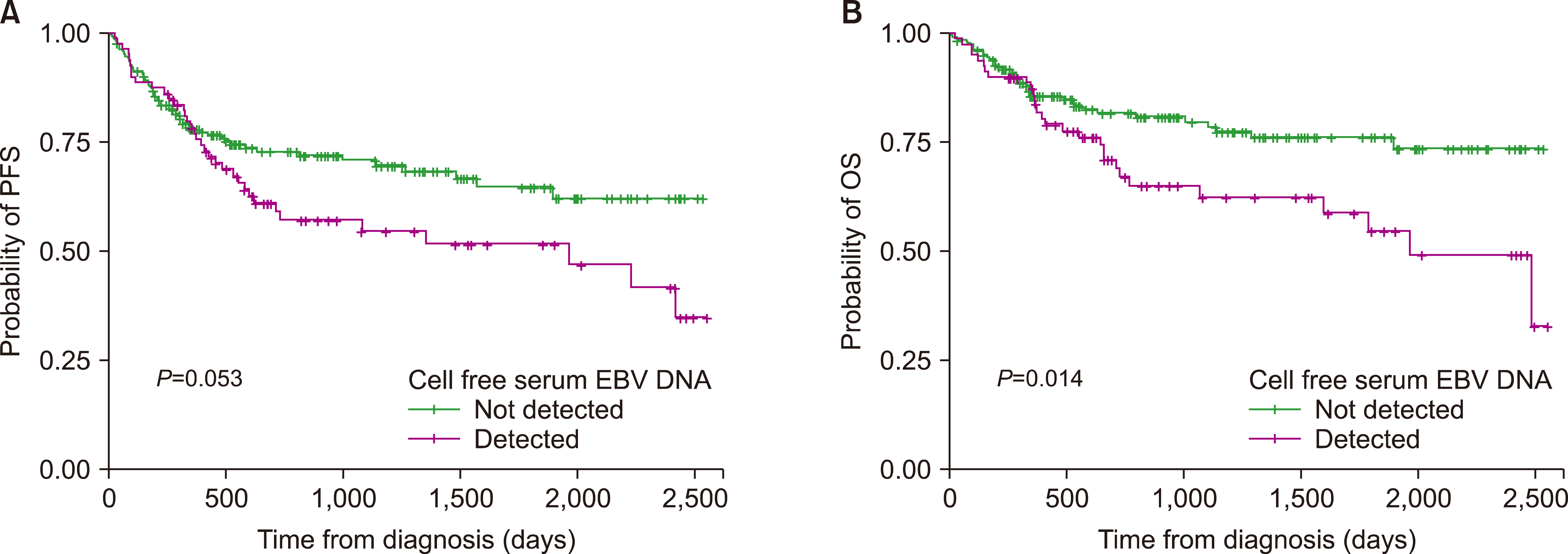
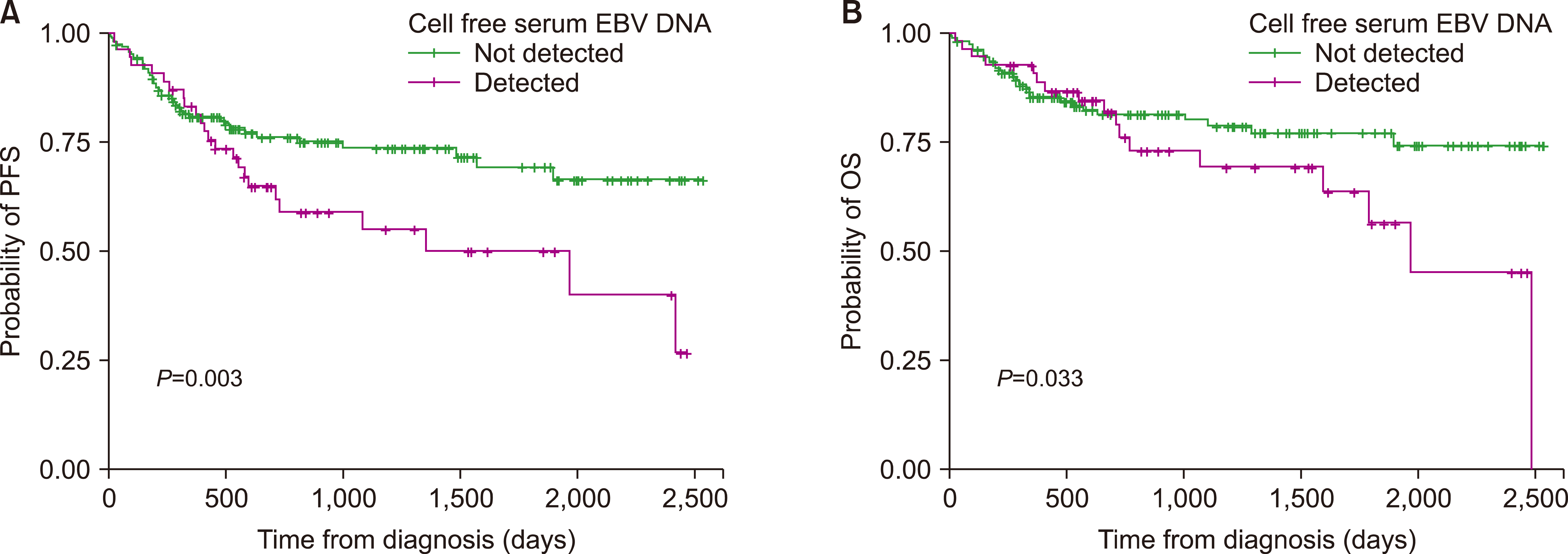
Serum EBV DNA was detected in 79 patients (30.0%). Patients with positive serum EBV tended to be older (P=0.090), and the proportion of T-cell lineage lymphomas was higher than that of B-cell lymphomas (P=0.003). EBV positivity was significantly associated with more advanced disease based on the Ann Arbor staging system (P=0.008) and the International Prognostic Index (P=0.009). EBV positivity was also associated with higher disease relapse (P=0.038) and death rates (P=0.005). EBV-positive lymphomas further showed inferior long-term survival outcomes in terms of progression-free survival (PFS) (P=0.053) and overall survival (OS) (P=0.014). In the subgroup analyses, serum EBV positivity was a significant prognostic factor for patients with B-cell lineage lymphomas in terms of PFS (P=0.003) and OS (P=0.033).
Conclusion
We demonstrated that cell-free serum EBV DNA status at the time of diagnosis has potential as a prognostic biomarker for patients with newly diagnosed malignant lymphomas.
Keywords: Epstein‒Barr virus, Lymphoma, Prognosis, Biomarker
INTRODUCTION
Epstein–Barr virus (EBV) is a human herpesvirus that is widely disseminated in humans, with approximately 90% of adults testing EBV seropositive. EBV persists asymptomatically for a lifetime as a latent EBV infection and is known to be linked to a variety of malignancies, including lymphomas [
1,
2]. The World Health Organization (WHO) classified EBV as an oncovirus in 1997 [
3].
EBV-associated lymphomas are a heterogeneous hematologic malignancy group that shares features of latent EBV infection within cancer cells. In general, EBV preferentially infects B-lymphocytes, and thus primarily occurs as B-cell lymphomas, such as Burkitt lymphoma, diffuse large B-cell lymphoma (DLBCL), and Hodgkin lymphoma (HL), although any type of EBV-associated lymphoma can develop [
1].
EBV has been shown to be a potential biomarker for predicting the prognosis and therapeutic targets in gastric cancer [
4]. Recent studies have been conducted to identify the contribution of latent EBV infection to disease development and prognosis in lymphomas. In a study of 437 patients with HL, EBV positivity was a significant prognostic factor affecting long-term survival [
5]. Park
et al. [
6] demonstrated that patients with EBV-positive DLBCL had poorer clinical outcomes. However, most studies analyzed lymphoid tissues and considered EBV positivity based on EBV-encoded RNA (EBER) using the
in situ hybridization (ISH) technique. Although some studies have shown the potential role of serum EBV viral load as a biomarker to determine disease burden, the clinical usefulness of cell-free serum EBV DNA in lymphomas has not been fully established [
7,
8].
The detection and quantification of serum EBV DNA has recently become possible using real-time quantitative polymerase chain reaction (RQ-PCR) using peripheral blood, which can be easily and safely obtained from patients to monitor EBV load. Therefore, the present study analyzed cell-free serum EBV DNA to identify its prognostic role in patients with newly diagnosed lymphomas.
MATERIALS AND METHODS
Patients
This study retrospectively reviewed 567 patients who were diagnosed with lymphoma between January 2014 and July 2020. Patients were enrolled according to the following criteria: i) pathologically confirmed lymphomas according to World Health Organization criteria [
9], ii) age over 18 years, iii) serum EBV DNA measurement using PCR prior to first-line therapy, and iv) receipt of curative standard chemotherapy. A total of 263 patients met these criteria and were included in this study. Patient records on medical history, age, sex, pathological results, treatment method, response, and survival were reviewed using electronic medical records (EMR). We predicted the prognosis of the enrolled patients according to the Lugano modification of the Ann Arbor staging system and the International Prognostic Index (IPI) [
10]. Treatment response was evaluated using the Lugano classification. This study was approved by the Institutional Review Board of KNUH.
Measurement of cell-free serum EBV DNA
To determine the clinical significance of serum EBV DNA, we retrospectively reviewed the data from the EMR. For the detection and quantification of serum EBV DNA at the time of diagnosis, EBV-specific RQ-PCR assays were conducted in the KNUH molecular microbiology laboratory using the Real-Q EBV DNA Quantification Kit (BioSewoom, Seoul, Korea) which was approved by the Korean Ministry of Food and Drug Safety. It targets the Epstein-Barr nuclear antigen 1 gene and utilizes the TaqMan probe-primer system. Nucleic acid was extracted using the MagNA Pure 96 system (Roche Diagnostics, Mannheim, Germany) and real-time PCR was carried out using the 7500 Fast Real-Time PCR system (Applied Biosystems, Carlsbad, CA, USA). The analytical measurement range of the Real-Q EBV DNA Quantification Kit ranges from 125 copies/mL to 1.0×1011 copies/mL, and a serum EBV DNA of ≥125 copies/mL was defined as positive (reference value <125 copies/mL).
Statistical analyses
Categorical variables were summarized as counts with proportions, and continuous variables were reported as medians with ranges. Progression-free survival (PFS) was calculated from the date of diagnosis to either the date of relapse, progression of disease, death from any cause, or last follow-up. Overall survival (OS) was measured from the date of diagnosis to the date of death from any cause or the date of the last follow-up. The Kaplan–Meier method was used to analyze the PFS and OS. Survival curves were compared using log-rank tests. The Cox regression model was used to identify factors affecting long-term survival. Factors with a
P-value of <0.1 in the univariate analysis were then included in the multivariate analysis. The hazard ratio (HR) and 95% confidence interval (CI) were estimated for each factor. Statistical significance was set at
P<0.05. The data of this retrospective study were analyzed using R statistical software program version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria; available at
http://www.r-project.org).
RESULTS
Patient characteristics
In this study, 263 patients with lymphoma were analyzed. The patient characteristics are summarized in
Table 1. The median age was 62 years (range, 18–87 yr) at the time of diagnosis and 141 (53.6%) patients were men. DLBCL was the most frequently diagnosed lymphoma, accounting for 68.1% of the cases. A small number of other types of lymphomas were also included. The Ann Arbor staging system and IPI prognoses were well stratified (
Supplementary Fig. 1). Serum EBV DNA was detected in 79 patients (30.0%). Forty-two patients with DLBCL (23.5%) were EBV positive. Among the serum EBV-positive cases, cases of mantle cell lymphoma (62.5%), natural killer/T-cell lymphoma (57.1%), angioimmunoblastic T-cell lymphoma (47.4%), and peripheral T-cell lymphoma (PTCL, 53.8%) tended to be relatively high (
Table 2).
Relevance of serum EBV positivity with regard to clinicopathologic factors
Serum EBV-positive patients tended to be older than those who were negative (
P=0.090), whereas sex was less relevant. The proportion of T-cell lineage lymphomas was higher in the EBV-positive group (
P=0.003). Serum EBV positivity was significantly associated with a more advanced disease based on the Ann Arbor staging system (
P=0.008) and IPI (
P=0.009). Patients who were EBV positive at the time of diagnosis had higher rates of disease relapse (
P=0.038) and death (
P=0.005) (
Table 3).
Clinical outcomes and impact of serum EBV positivity on survival
With a median follow-up duration of 23.3 months, 67 (25.5%) patients were primarily refractory to first-line therapy or had disease relapse, whereas 65 (24.7%) died. The 2-year PFS and OS rates were 67.5% and 76.9%, respectively. In the analysis of all enrolled patients, serum EBV-positive lymphoma at diagnosis showed inferior long-term survival outcomes in terms of PFS (
P=0.053) and OS (
P=0.014) (
Fig. 1). In the subgroup analyses, serum EBV positivity was a significant prognostic factor for patients with B-cell lineage lymphomas in terms of PFS (
P=0.003) and OS (
P=0.033) (
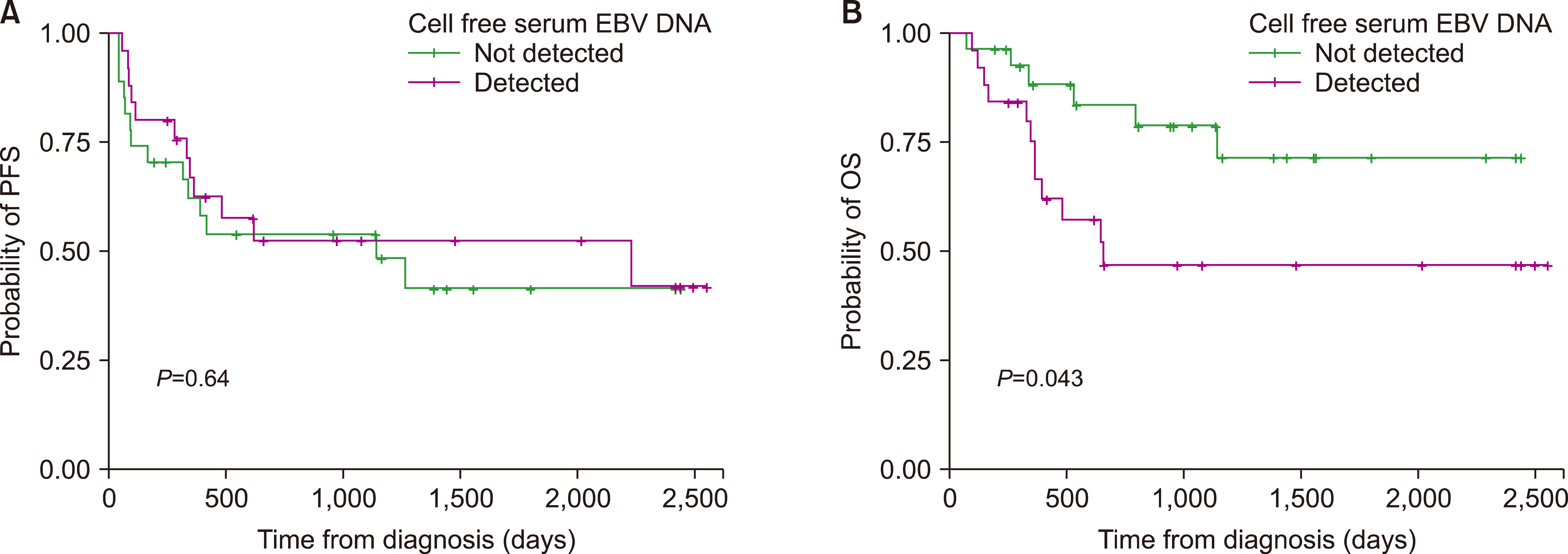
Fig. 2). In T-cell lymphomas, EBV-positive patients showed a more inferior OS; however, EBV positivity was not a significant factor affecting PFS (
Fig. 3).
Independent prognostic factors affecting long-term outcomes
In the univariate survival analysis, the subtypes of lymphoma (B-cell lymphomas vs. T-cell lymphomas), prognostic risk group (high-intermediate, high risk vs. low, low-intermediate risk), and serum EBV positivity were significantly associated with PFS (HR, 0.528; 95% CI, 0.326–0.853;
P=0.009; HR, 3.018; 95% CI, 1.699–5.358;
P<0.001; and HR, 1.354; 95% CI, 1.102–1.723;
P=0.045; respectively) and OS (HR, 0.618; 95% CI, 0.350–1.092;
P=0.097; HR, 4.389; 95% CI, 2.157–8.929;
P<0.001; HR, 1.647; 95% CI, 0.990–2.738;
P=0.054; respectively). Multivariate survival analysis revealed that B-cell lymphoma was significantly associated with better PFS (HR, 0.503; 95% CI, 0.318–0.795;
P=0.003), while high- and high-intermediate-risk groups and positive serum EBV were independent poor prognostic factors for PFS (HR, 3.083; 95% CI, 2.022–4.699;
P<0.001; HR, 1.211; 95% CI, 1.097–1.582;
P=0.048; respectively) and OS (HR, 4.352; 95% CI, 2.612-7.252;
P<0.001; HR, 1.643; 95% CI, 1.108–2.705;
P=0.050; respectively) (
Table 4).
DISCUSSION
Latent EBV infection is known to be associated with the pathogenesis of several lymphomas. Moreover, EBV positivity based on ISH to the EBER has shown potential as a clinically significant prognostic marker [
6,
11]. Although cell-free serum EBV status also has the potential to predict the prognosis of patients with lymphomas, critical studies on clinical implications are still lacking. In this study, we analyzed 263 newly diagnosed patients with lymphoma who had received curative standard treatment, and serum EBV DNA was detected in 79 patients. Although our data included various lymphoma subtypes, patients who were serum EBV DNA positive prior to the first treatment showed significantly poor prognosis regardless of age and sex. Moreover, serum EBV status was correlated with the Ann Arbor staging system and IPI, suggesting that serum EBV DNA status at the time of diagnosis might be an independent prognostic indicator.
Previous studies reported that approximately 10% of patients with DLBCL had a positive EBER ISH status [
6,
12]. However, the frequency of serum EBV DNA positivity in pretreated patients with lymphoma has not been elucidated. A retrospective study reported that 25% of PTCL patients showed elevated serum EBV DNA load, which was associated with shorter survival outcomes [
13]. In this study, 30% of the patients had a positive serum EBV status. However, except for DLBCL, which had a 23.5% positive rate, the proportion of patients with other lymphoma subtypes was too small to be statistically significant. Therefore, larger sample sizes for each lymphoma subtype are required. Meanwhile, serum EBV positivity is more prevalent than tissue-based positive EBER ISH status according to previous studies [
13,
14]. The frequency of serum EBV positivity may be higher than that of lymphoma tissue because >90% of the population is EBV seropositive regardless of lymphoma diagnosis. Therefore, validation studies confirming the clinical usefulness and standardization of serum EBV measurements are warranted. Given that the EBV status of patients with lymphoma shows potential as a novel biomarker for prognosis, serum EBV DNA status may be more clinically valuable. Furthermore, serum biomarkers can be evaluated after continuous, easy, and safe treatment is established as a concept of minimal residual disease.
While the present study showed the potential role of serum EBV status in predicting long-term clinical outcomes, the results should be cautiously interpreted due to certain limitations. First, data in this study were retrospectively analyzed. Second, this study included heterogeneous histologic subtypes, and no detailed information was provided on the treatments. In addition, the sample size, except for DLBCL, was too small to identify clinical significance. Third, we could not clarify the association between tissue and serum EBV infection. Lastly, comparison survival outcomes based on the viral load in serum EBV DNA and cut-off values should be explored in future studies.
In conclusion, this study demonstrated cell-free serum EBV status at the time of diagnosis as a potential prognostic marker for newly diagnosed lymphomas. B-cell lymphomas were shown to be more relevant than T-cell lymphomas in this study; however, well-designed studies with large samples for each lymphoma subtype are warranted to clarify the clinical impact of serum EBV status.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download