TO THE EDITOR: Most patients with acute B-lymphoblastic leukemia (ALL) present with cytopenia, lymphadenopathy, and hepatosplenomegaly [1]. Osteolytic bone lesions are common in plasma cell myelomas and metastatic tumors, but they are rare in ALL patients, especially in adults [2]. Thus, the diagnosis is difficult, and an accurate diagnosis may be delayed when osteolytic lesions are the primary presentation in ALL. Here, we report a case of adult B-ALL initially presenting solely as multiple osteolytic bone lesions and discuss its clinical significance through a literature review. To our knowledge, this is the oldest patient case so far with infrequent and rare presentation of adult B-ALL.
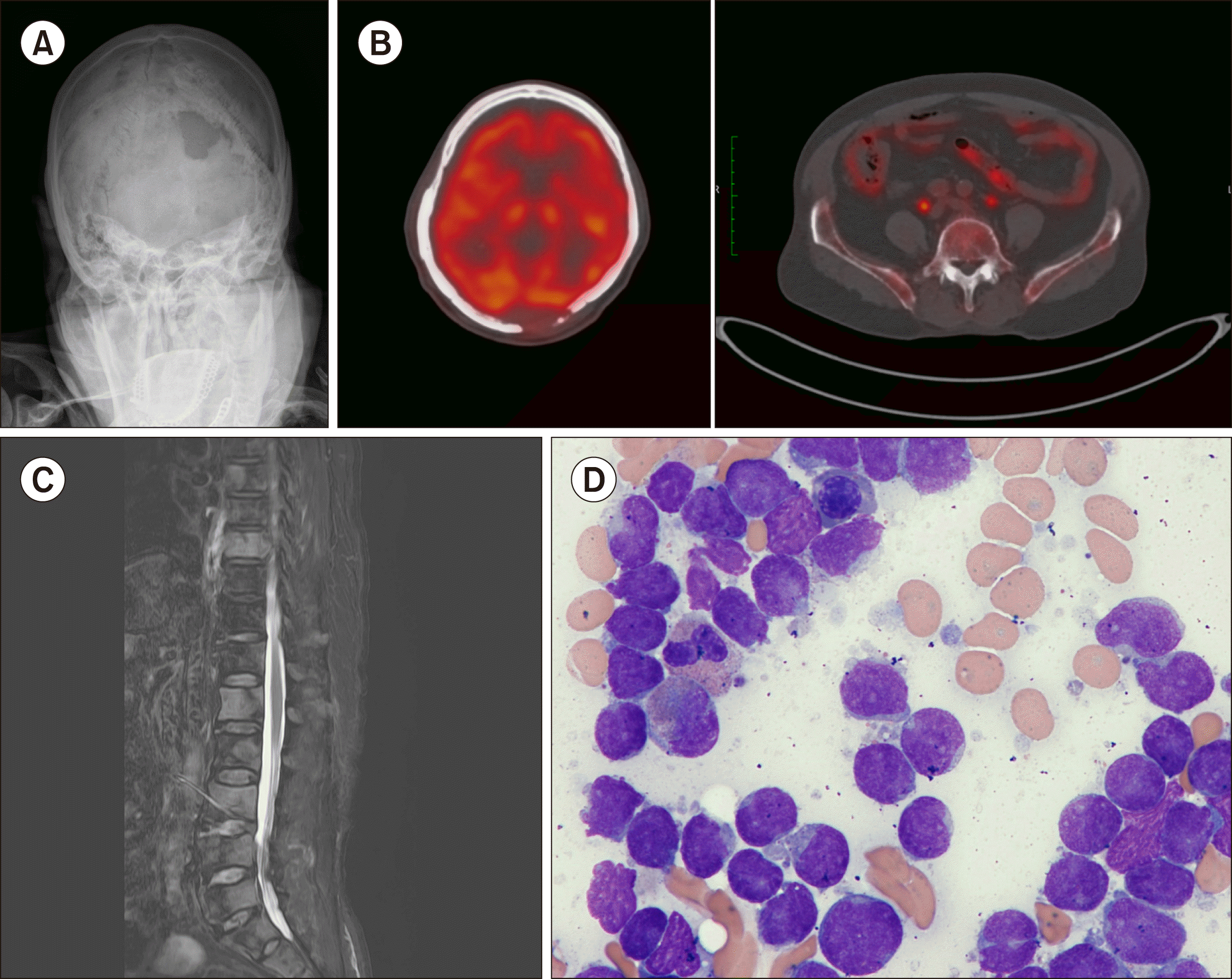
A 78-year-old man was admitted for back pain. The magnetic resonance image of the spine, bone scan, and positron emission tomography-computed tomography showed malignant bone infiltration in the skull, whole vertebrae, ribs, pelvic bones, and a concurrent pathologic compression fracture in the thoracic vertebra. Simple radiography of the skull revealed multiple osteolytic lesions, suggesting the involvement of plasma cell myeloma (Fig. 1). A physical examination and computed tomography revealed the absence of hepatosplenomegaly and lymphadenopathy. The complete blood count at admission showed white blood cells, 6.06×109/L; hemoglobin, 10.2 g/dL; and platelets, 132×109/L. A peripheral blood smear demonstrated no circulating blasts or abnormal cells. The differential counts of leukocytes were as follows: neutrophil, 79%; lymphocytes, 12%; and monocytes, 9%. Serum total protein and albumin levels were 6.9 g/dL (reference range, 5.8–8.1 g/dL) and 4.4 g/dL (reference range, 3.1–5.2 g/dL), respectively. The serum creatinine level was 0.98 mg/dL (reference range, 0.5–1.2 mg/dL); lactate dehydrogenase level, 324 IU/L (reference range, 0-250 IU/L); and, calcium level, 9.98 mg/dL (reference range, 8.0–10.5 mg/dL). Serum and urine protein electrophoreses and immune-fixation electrophoresis showed no monoclonal bands, and the levels of free light chains in serum and urine were normal. Bone marrow aspiration showed 70.4% leukemic blasts with 75% cellularity in the biopsy (Fig. 1). The leukemic blasts expressed CD10, CD13, CD19, CD20, CD22, CD33, CD34, TdT, and cytoplasmic and surface immunoglobulins, consistent with B-cell lymphoblastic leukemia, a common cell type with aberrant expression of CD13 and CD33. A chromosomal analysis revealed 46,XY [20], and there were no detectable genetic abnormalities in the molecular test. He received radiation therapy only to the thoracic and lumbar spines because of his poor general condition. After radiation therapy, he was transferred to another hospital for conservative treatment.
One of the most important factors to be considered while evaluating lytic bone lesions is the age of the patient. In the elderly, differential diagnoses for multiple osteolytic lesions include histiocytosis, enchondroma, fibrous dysplasia, and hyperparathyroidism. In addition, metastatic malignancy and plasma cell myeloma should always be considered in middle-aged and elderly patients [3].
Multiple osteolytic bone lesions are a very rare presentation of adult B-ALL, and there have been only a few sporadic reports [2, 4-8]. Most reported patients showed diffuse and multiple osteolytic lesions, normal white blood cell counts without circulating blasts, mildly decreased hemoglobin and/or platelet count, and no organomegaly and/or lymphadenopathy [2, 4, 6-8]; these reports are consistent with those of our patient. The immunophenotype of leukemic blasts in our patient was a common cell type (CD19 and CD10 positive), a finding similar to those of other studies [2, 4, 8]. Furthermore, some studies have reported hypercalcemia accompanying elevated parathyroid related protein (PTHrP) [4-6]. However, our patient did not show hypercalcemia, which is consistent with a finding of another study [2]. A previous study found an association between t(17;19) translocation and osteolytic lesions in childhood ALL [9]; however, no significant genetic abnormalities have been reported in adult ALL patients with osteolytic lesions.
The mechanisms underlying osteolytic bone lesions in ALL are not well known. Some cases of osteolytic lesions caused by malignancy showed concurrent hypercalcemia possibly due to local bone destruction by direct invasion of the tumor or by increased osteoclastic activity through tumor-secreting factors such as PTHrP, interleukin (IL)-3, IL-6, and tumor necrosis factors [2, 4]. Among them, PTHrP production by B-ALL blasts has been considered a main cause of osteolytic lesions [2, 7]. In our case, however, we did not check the level of humoral factors; thus, we could not investigate the exact mechanism in our patient.
The prognostic effect of osteolytic lesions in ALL remains unclear. Although a few studies have reported improved survival in pediatric patients with ALL having osteolytic lesions due to better responsiveness to steroids [2, 10], there are no related studies on adults. Early suspicion and diagnosis may be the most crucial factors related to increased survival in adult patients with ALL with osteolytic bone lesions.
In conclusion, we report a very rare case of adult B-ALL that initially presented as multiple osteolytic lesions. Despite these atypical initial presentations, early recognition and treatment are essential for improving outcomes.
REFERENCES
1. Swerdlow SH, Campo E, Harris NL, editors. 2017. WHO classification of tumours of haematopoietic and lymphoid tissues. Revised 4th ed. IARC Press;Lyon, France:
2. Angsubhakorn N, Suvannasankha A. 2018; Acute lymphoblastic leukaemia with osteolytic bone lesions: diagnostic dilemma. BMJ Case Rep. 2018:bcr2018225008. DOI: 10.1136/bcr-2018-225008. PMID: 30100571. PMCID: PMC6088299.

3. Li X, Wu N, Zhang W, Liu Y, Ming Y. 2020; Differential diagnostic value of 18F-FDG PET/CT in osteolytic lesions. J Bone Oncol. 24:100302. DOI: 10.1016/j.jbo.2020.100302. PMID: 32760643. PMCID: PMC7393436.
4. Granacher NCP, Berneman ZN, Schroyens W, Van de Velde ALR, Verlinden A, Gadisseur APA. 2017; Adult acute precursor B-cell lymphoblastic leukemia presenting as hypercalcemia and osteolytic bone lesions. Exp Hematol Oncol. 6:9. DOI: 10.1186/s40164-017-0071-8. PMID: 28401025. PMCID: PMC5387187.

5. Chung SW, Kim S, Choi JR, Yoo TH, Cha IH. 2011; Osteolytic mandible presenting as an initial manifestation of an adult acute lymphoblastic leukaemia. Int J Oral Maxillofac Surg. 40:1438–40. DOI: 10.1016/j.ijom.2011.01.013. PMID: 21723711.

6. Verma SP, Dubashi B, Basu D, Dutta TK, Kar R. 2014; A rare case of adult acute lymphoblastic leukemia presenting with paraparesis and multiple osteolytic lesions. Indian J Hematol Blood Transfus. 30(Suppl 1):24–6. DOI: 10.1007/s12288-012-0221-4. PMID: 25332525. PMCID: PMC4192164.

7. Fukasawa H, Kato A, Fujigaki Y, Yonemura K, Furuya R, Hishida A. 2001; Hypercalcemia in a patient with B-cell acute lymphoblastic leukemia: a role of proinflammatory cytokine. Am J Med Sci. 322:109–12. DOI: 10.1097/00000441-200108000-00009. PMID: 11523624.

8. Mahmood K, Ubaid M, Taliya Rizvi S. 2017; Multiple osteolytic lesions causing hypercalcemia: a rare presentation of acute lymphoblastic leukemia. Case Rep Med. 2017:2347810. DOI: 10.1155/2017/2347810. PMID: 28798774. PMCID: PMC5536143.

9. Inukai T, Hirose K, Inaba T, et al. 2007; Hypercalcemia in childhood acute lymphoblastic leukemia: frequent implication of parathyroid hormone-related peptide and E2A-HLF from translocation 17;19. Leukemia. 21:288–96. DOI: 10.1038/sj.leu.2404496. PMID: 17183364.

10. Heinrich SD, Gallagher D, Warrior R, Phelan K, George VT, MacEwen GD. 1994; The prognostic significance of the skeletal manifestations of acute lymphoblastic leukemia of childhood. J Pediatr Orthop. 14:105–11. DOI: 10.1097/01241398-199401000-00021. PMID: 8113359.

Fig. 1
Simple radiography of the skull (A), positron emission tomo-graphy-computed tomography (B), and magnetic resonance imaging showing multiple osteolytic lesions in skull (C), vertebrae, rib, both pelvic bones, and a concurrent pathologic compression fracture in the thoracic vertebra; leukemic blasts observed in the bone marrow aspirate (D).




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download