INTRODUCTION
Platelet aggregometry has been employed for the detection, diagnosis, and monitoring of qualitative platelet disorders since the early 1960s [
1]. The historic gold standard, optical-based light transmission aggregometry (LTA) is widely used for platelet aggregation studies, measuring platelet aggregation in response to several agonists such as adenosine diphosphate (ADP), collagen, and epinephrine in platelet-rich plasma (PRP) [
2]. Response to different agonists provides insight into the activation and/or aggregation of individual platelet pathways. However, problems are associated with LTA, necessitating the need for better techniques [
3]. Flow cytometry, a major technological advancement, is used to examine a plethora of platelet defects and features [
3,
4]. Flow cytometric platelet aggregation (FCA) assay allows the detection of platelet aggregates based on the principle that differently labeled washed platelets or platelets in whole blood from the same donor form double-colored platelet aggregates when stimulated by agonists under appropriate conditions [
5-
7]. Few published studies have demonstrated the clinical applicability of FCA assay; studies comparing the results of LTA and FCA assay in bleeding patients for diagnosing underlying platelet function defects (PFD) are limited. Hence, this study aimed to evaluate the clinical utility of FCA assay for detecting PFDs and to correlate the results obtained with those of LTA.
MATERIALS AND METHODS
Patients and healthy controls
Patients clinically suspected of having a PFD who were examined in the Adult and Pediatric Hematology Clinic between July 2017 and June 2018 were included. Patients aged >1 year; with normal prothrombin time (PT), activated partial thromboplastin time (aPTT), fibrinogen levels, von Willebrand factor (VWF) antigen levels, and ristocetin cofactor activity; and with a negative urea clot solubility test were included. Bleeding history was determined using a standard bleeding assessment tool (BAT) (ISTH/SSC) [
8], and BAT scores were calculated. LTA and FCA assay were performed simultaneously in patients and healthy controls. All participants reported abstinence from antiplatelet therapy/anti-inflammatory drug use for the past 7 days. The study was approved by the Institutional Ethics Review Board (IEC reference no. NK/3349/DM) and was performed in accordance with the principles of the 1964 Declaration of Helsinki and its revisions. Detailed informed consent was obtained from all participants or the child’s guardians/parents.
Blood sample
Approximately 10 mL of peripheral blood was collected using a wide-bore 21-gauge needle into a Vacutainer tube [Becton Dickinson (BD), Franklin Lakes, NJ, USA] containing 3.2% (0.109 M) trisodium citrate. PRP was prepared by centrifuging the citrate-anticoagulated whole blood at 170–200 g for 10–15 min at 20°C. Platelet poor plasma (PPP) was obtained by centrifuging the remaining blood sample at 2,000 g for 20 min. For testing, 1,500 mL of PRP was utilized for the LTA and 200 mL for the FCA assay.
LTA
LTA was performed using a CHRONO-LOG Model 700 aggregometer in a four-channel configuration (Havertown, PA, USA) using PRP; the optical changes after agonist (all from CHRONO-LOG Corporation, Havertown, PA, USA) stimulation were monitored on a computer using the AGGRO/LINK 8 software. Approximately 5 mL of ADP (1 mM) was added to 500 mL of PRP to attain a final concentration of 10 µM ADP, while 2 mL of collagen (1 mg/mL) and 5 mL of ristocetin (125 µg/mL) were added to 500 mL of PRP to attain the final concentrations of 2 µg/mL collagen and 1.25 mg/mL ristocetin. After adding the agonists to the cuvettes, the curve characteristics were examined, and the percentage maximum amplitude of response (% maxA of response) was recorded.
FCA assay
FCA assay was performed according to the strategy adopted by De Cuyper
et al. [
5]. Briefly, 100 mL of PRP two separate BD Falcon tubes were incubated for 15 min with 5 mL of anti-CD31 FITC-labeled and anti-CD31 PE-labeled antibodies (BD Biosciences, San Jose, NJ, USA). The sample was washed twice with 500 mL of sequestrene buffer and resuspended in HEPES buffer medium containing 20% AB+ CPD plasma. Differently labeled washed platelets were mixed in a 1:1 ratio (500 mL) and supplemented with AB+ CPD plasma (100 mL) and 20 mM (0.9 mL) D-phenylalanyl-L-prolyl-L-arginine chloromethyl ketone (thrombin inhibitor, PPACK, Calbiochem, Darmstadt, Germany). The samples were incubated at 37°C with shaking (Thermomixer comfort, Eppendorf, Hamburg, Germany) at 700 rpm for 10 min. After incubation, 3 mM calcium chloride was added. A portion of the unstimulated sample (50 mL) was fixed by adding nine volumes of 0.5% formaldehyde in phosphate-buffered saline (450 mL), which served as a baseline (t=0 min). The remaining unstimulated platelet mix was distributed into three microcentrifuge tubes containing 250 mL of each sample. Agonist concentration was selected by performing a dose-response analysis of an ideal dose, resulting in maximal platelet aggregation. The platelets were activated at 37°C with ADP, collagen, and ristocetin (final concentrations: 244 mM, 10 mg/mL, and 1.5 mg/mL, respectively) while shaking at 1,000 rpm. At 10 min (t=10), a portion of the sample from each of the agonist-stimulated platelet mix (50 mL) was again fixed. Fixed samples were measured using a flow cytometer (BD FACS Canto II) and analyzed with BD FACS DIVA software version 6.1. At least 20,000 events were acquired using a cytometer. The platelets were identified on a log-scaled forward scatter (FSC) and side scatter plot (SSC) and examined further on CD31-FITC and CD31-PE dot-plot combinations. A direct wet mount microscopic examination of the unstimulated platelet mix at t=0 min and agonist-stimulated platelet mix (t=10 min) was performed, and platelet aggregation was confirmed in the latter. Discrete platelets were detected in the unstimulated platelet mix at t=0 min, indicating non-activation or non-aggregation of platelets during preparation (
Supplementary Fig. 1). The percentage of double-colored events was derived from these parameters using the formula % double-colored events=[Q2/(Q1+Q2+ Q4)×100]. Representative images of the flow cytometric dot plots from healthy controls are shown in
Fig. 1. Various quality control measures were adopted to ensure the reliability and repeatability of the assay. The LTA and FCA assay results were compared between patients and controls.
Fig. 1
Flow cytometric platelet aggregation of a healthy control. Dot plots showing platelets in unstimulated (t=0 min) and ADP-stimulated state (t=10 min) in a healthy control (left and right, respectively). The platelets are gated on a log forward (FSC) and side scatter (SSC) (upper panel) and examined on CD31-FITC and CD31-PE combination (lower panel). The double-colored events (Q2) represent the platelet aggregates, Q1 represents the CD31-PE-labeled platelets, Q4 represents the CD31-FITC-labeled platelets, and Q3 represents the unstained events (plasma, debris, etc.). The % double-colored events in this control in unstimulated t=0 min and ADP-stimulated platelet mix at t=10 min are 1.8% and 41.4% respectively.

Statistical analysis
Statistical analysis was performed using GraphPad Prism version 8.4.2 for Windows (GraphPad Software, San Diego, CA, USA). The normality of data was assessed using Kolmogorov–Smirnov analysis. The PT, aPTT, and fibrinogen values were normally distributed; hence, the mean values were calculated. Data on age, BAT score, hemoglobin level, total leukocyte count, platelet count, VWF antigen levels, and ristocetin co-factor activity were skewed. Hence, the medians and ranges of these variables were calculated. The normal range of % maxA of response in LTA and the percentage of double-colored events in FCA assay to agonists ADP, collagen, and ristocetin were set using the results of healthy controls. The normal range of the % maxA of response to ADP, collagen, and ristocetin in LTA are expressed as mean±3SD; the percentage of double-colored events in ADP, collagen, and ristocetin-stimulated platelet-mix on FCA assay are expressed as mean±SD. Results below the reference range indicated an impaired response to the agonist tested. Cohen’s kappa coefficient was used as a statistical measure to identify the degree of agreement between the results of the LTA and FCA assay. All tests were two tailed, and a P-value of <0.05 was considered significant.
RESULTS
Demographic characteristics and baseline data
Twenty-four patients and 40 controls were included in the study. LTA and FCA assay were performed simultaneously in 22 patients and healthy controls using PRP. Only LTA data were available in 20 healthy controls, while FCA assay data were available in two thrombocytopenic patients. The patients’ median age and male-to-female ratio was 17 years (range, 3–72 yr) and 1:1.7, respectively. Two patients had consanguineous parents. Six patients had a history of blood transfusion.
A standardized ISTH-BAT questionnaire was used to assess bleeding, revealing an elevated BAT score (>3 in adult men, >5 in adult women, and ≥3 in pediatric patients; total score: 10) in 17 (77.3%) of 22 patients. The median BAT score was 4.5 (range, 3–11). The most commonly observed bleeding symptom was cutaneous bleeding (N=14, 63.6%), followed by menorrhagia (N=7, 31.8%), oral cavity bleeding (N=6, 27.7%), epistaxis (N=6, 27.3 %), minor wound bleeding (N=5, 22.7%), gastrointestinal bleeding (N=2, 9%), surgical bleeding (N=2, 9%), central nervous system bleeding (N=1, 4.5%), and muscle hematoma (N=1, 4.5%). A significant direct correlation was observed between a higher BAT score and cutaneous bleeding (
P=0.001).
Table 1 presents the patients’ demographic characteristics and baseline hematological parameters.
Table 1
Demographic and baseline laboratory data of the study patients.
|
Parameter |
Results |
|
N |
22a)
|
|
Median age in years (range) |
17 (3–72) |
|
Median BAT score (range) |
4.5 (3–11) |
|
PT in seconds: mean (±2SD) |
13 (±2.83) |
|
aPTT in seconds: mean (±2SD) |
30.9 (±6.85) |
|
Fibrinogen level in g/dL: mean (±2SD) |
3.17 (±1.43) |
|
Hemoglobin in g/L: median (range) |
113 (57–148) |
|
Total leucocyte count ×109/L: median (range) |
7.2 (2.7–113.5) |
|
Platelet count ×109/L: median (range) |
217 (123–425) |
|
VWF antigen assay: % median (range) |
123.05 (68.8–223.6) |
|
VWF GPIbR (RiCoF activity) assay: % median (range) |
109.3 (58.7–221.3) |

End results of the LTA and FCA assay
Data generated from two techniques [maxA of response to ADP, collagen, and ristocetin on LTA and the percentage of double-colored events in ADP-, collagen-, and ristocetin-stimulated platelet mix (t=10 min) on FCA assay] in healthy controls and patients were recorded (
Fig. 2). In the LTA, the normal ranges of the % maxA of response to ADP, collagen, and ristocetin among the healthy controls were 47.2–115.78%, 53.5–114%, and 69–107.4%, respectively. In the FCA assay, the percentages of double-colored events for ADP-, collagen-, and ristocetin-stimulated platelet mix among the healthy controls (N=22) were 20–48.4%, 16.1–21.1%, and 20.7–49.2% respectively. Results below the lower limit of the reference range were considered to indicate impairment/abnormalities.
Fig. 2
Scatter diagrams showing the distribution of test results. The % maximum amplitude of response to ADP, collagen, and ristocetin in light transmission aggregometry (A) and the % double-colored events of ADP-, collagen-, and ristocetin-stimulated platelet mix on flow cytometric platelet aggregation assay of healthy controls and patients are shown (B).

Substantial agreement of results between LTA and FCA assay
Among the 22 patients, normal platelet aggregation to all three agonists was noted in 16 (72.7%) and 14 (63.6%) patients by LTA and FCA assay, respectively. Abnormal platelet aggregation to at least one of the agonists was noted in six (27.2%) and eight (36.3%) patients by LTA and FCA assay, respectively.
Table 2 summarizes the comparison and agreement of the end results between the LTA and FCA assay. Collagen and ristocetin were the most common agonists to which an impaired response was noted in the LTA, as opposed to ADP in the FCA assay. Statistical quantification of inter-rater agreement showed a substantial agreement between LTA and FCA assay in terms of detecting PFDs (k=0.792). The measures of agreement (Kappa statistics) between the results of LTA and those of FCA assay for ADP, collagen, and ristocetin were 0.861 (almost perfect agreement), 0.741 (substantial agreement), and 0.179 (slight agreement), respectively.
Table 2
Agreement between the results of light transmission aggregometry and flow cytometric platelet aggregation assay performed simultaneously in 22 patients.
|
Technique |
Flow cytometric platelet aggregation |
|
Light transmissi on aggregometry |
|
Normal |
Abnormal |
|
Normal |
14 |
2 |
|
Abnormal |
0 |
6 |

Glanzmann’s thrombasthenia (GT)-like pattern in LTA and FCA assay
LTA identified four patients with a pattern consistent with that of GT. They exhibited impaired ADP and collagen aggregation, but showed minimally impaired response to ristocetin (
Fig. 3A). These patients served as negative controls for FCA assay (
Fig. 3B). The FCA assay showed an impaired response to ADP in all four patients, impaired response to collagen in three patients (
Fig. 3B, patient 2), and abnormal response to ristocetin in one patient (
Fig. 3B, patient 4). Results of flow cytometry to evaluate the platelet surface glycoprotein marker expression in patients 1, 2, and 3 (
Fig. 3) revealed markedly reduced or absent aIIbb3 receptor (CD41a/CD61) with normal CD42b expression levels (GpIb), characteristic of GT. Patient 4 (
Fig. 3A) showed an LTA pattern consistent with that of GT, but had normal levels of CD42b, CD41a, and CD61 on flow cytometry. This patient had an impaired response to all three agonists even on FCA assay (
Fig. 3B), but had markedly impaired response to collagen (2.2%) compared with that of the other three patients with GT (14.31%, 11.6%, and 30.7%).
Fig. 4 illustrates the FCA plots of a patient (
Fig. 3, patient 1) showing an LTA pattern consistent with that of GT.
Fig. 3
Scatter plots showing the test results of patients 1, 2, 3, and 4 with a GT-like pattern on light transmission aggregometry (LTA).
(A) % aggregation of LTA and
(B) % aggregation of flow cytometric platelet aggregation to agonists ADP, collagen, and ristocetin are plotted. Note that patient 1 in the
Fig. 3A has superimposed ADP and collagen values.
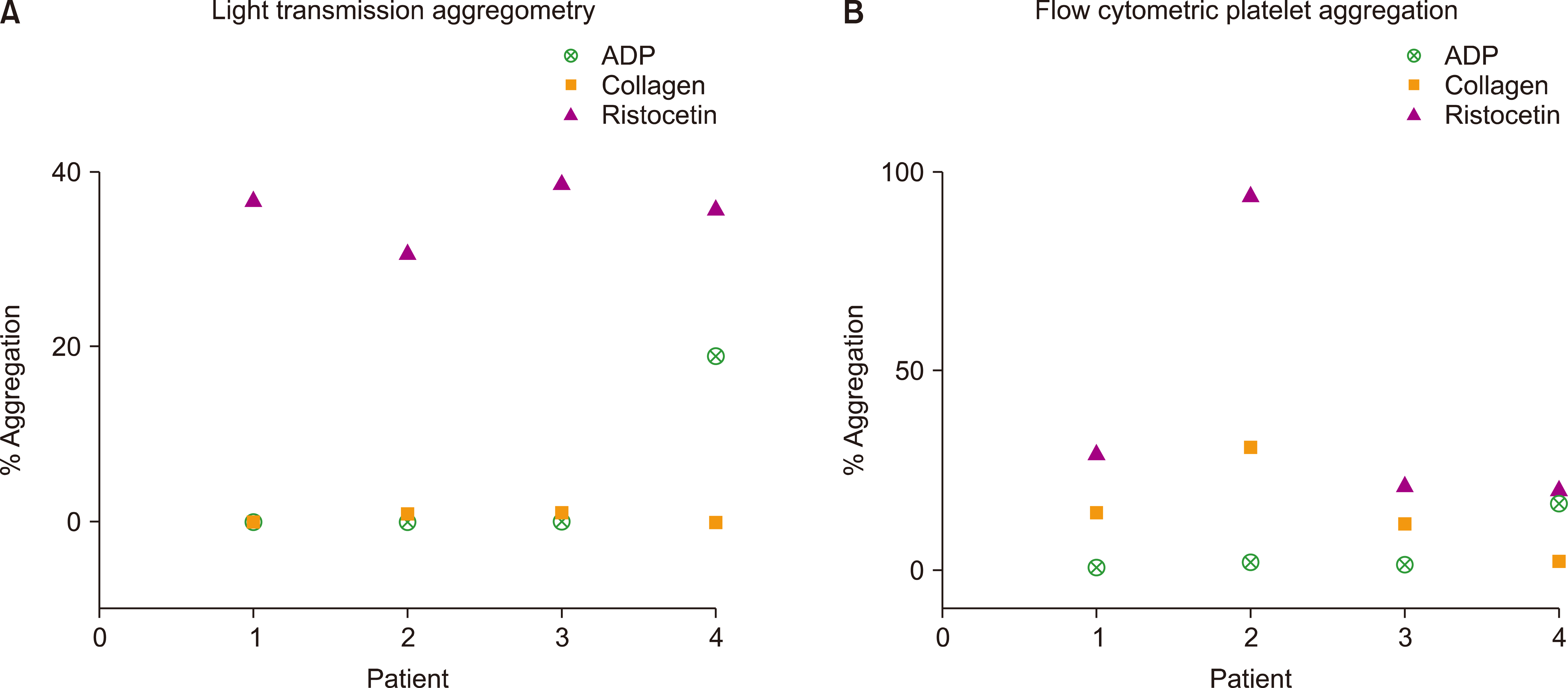

Fig. 4
Flow cytometric platelet aggregation assay of a patient with Glanzmann thrombasthenia (GT). Dot plots showing platelets in unstimulated (t=0 min) and agonist-stimulated state (t=10 min) in a GT patient. The % double-colored events in this patient in unstimulated t=0 min and ADP-, collagen-, and ristocetin-stimulated platelet mix at t=10 min are 0.6% and 0.7%, and 14.3% and 29.9% respectively.

Comparison of performance characteristics between FCA assay and LTA
The FCA assay (1%) had better coefficient of variation (CV) than the LTA (7%), indicating good reproducibility of the test results of FCA. A few patients were examined on two separate occasions (1 month apart) to assess the repeatability of FCA assay. No change was observed in the response category from normal to abnormal, or vice versa, indicating good repeatability of FCA.
Association between FCA assay and platelet count and its applicability in thrombocytopenic patients
To examine the applicability of FCA to thrombocytopenic patients, dilution experiment was carried out, which involved the dilution of PRP with PPP, decreasing the platelet concentration from 280×109/L to 20×109/L. Similar results were demonstrated in the baseline and diluted samples, indicating that the performance of FCA assay was independent of platelet counts. To confirm the above inference, two patients with significant bleeding, clinically suspected to have idiopathic thrombocytopenic purpura with a platelet count of ∼20×109/L and 41×109/L, respectively, underwent platelet function testing. As LTA was inapplicable due to the low platelet count, FCA assay was performed. The FCA assay showed impaired response to collagen in both patients, suggesting a possible functional platelet deficiency in both patients.
DISCUSSION
We tested the applicability and reproducibility of a novel FCA technique, standardized in our laboratory, by incorporating various test validation methods. The protocol was modified from a previously described technique wherein anti-CD31-labeled antibody was used in PRP for platelet labeling instead of utilizing cytoplasmic dyes and whole blood [
5]. CD31 (endoCAM/PECAM) is an integral membrane glycoprotein expressed on the surface of platelets and myeloid cells such as neutrophils and monocytes [
9,
10]. Platelet-specific CD41/CD61 and CD42 were not utilized for labeling, as they might be absent or their expression in patients with inherited platelet disorders might be reduced. To maintain uniformity in the approach, CD31 was utilized as a platelet marker, preferred for FCA assay [
5]. The use of log FSC and SSC gating strategies effectively excluded the other cells, enabling the evaluation of CD31 expressing platelets only.
Table 3 summarizes studies that specifically examined platelet aggregation using flow cytometry.
Table 3
Studies utilizing the flow cytometric platelet aggregation technique.
|
Research study |
Sample size |
Patients |
Platelet labelling |
N of platelet agonists tested |
Platelet agonists used |
Remarks |
|
De Cuyper et al. (2013) [5] |
NA |
Human and mouse platelets |
1.CD31 for labelling of platelets in whole blood
2.CFSE and PKH26 dyes for washed platelets |
3–4 |
PMA, type I collagen, Aggretin A, or ristocetin |
Platelet aggregation using flow cytometry can be performed with small starting volume or low platelet count. |
|
van Bladel et al. (2014) [6] |
33 |
Pediatric chronic ITP patients |
CFSE and PKH26 dyes for washed platelets |
2 |
PMA or ristocetin |
Decreased platelet function is seen in patients with severe bleeding phenotype. |
|
Vinholt et al. (2017) [7] |
20 |
TCP patients diagnosed with acute myeloid leukemia or myelodysplastic syndrome |
CAMU and CV450 dyes for washed platelets |
3 |
Collagen-related peptide TRAP and ADP |
Platelet aggregation assay is applicable in TCP patients to identify a bleeding risk. |
|
Present study (2020) |
24 |
Bleeding patients suspected to have a platelet function defect including two TCP patients |
CD31 for washed platelets |
3 |
ADP, type-I collagen, Ristocetin |
FCA is a potential technique for identification of PFD comparable to LTA, with applicability in TCP patients. |

We found a substantial inter-rater agreement between the LTA and the FCA assay in detecting a PFD. Among the 22 patients, 20 (90.9%) were identified in similar categories using both test methods. Fourteen patients showed normal results, while six patients exhibited a PFD on both techniques. Among the six patients, four with a GT-like pattern on LTA (impaired response to ADP, collagen, and ristocetin) served as ideal negative controls for FCA assay. All four patients showed an impaired response to one or more agonist on FCA assay, but with a variable pattern (impaired response to ADP, N=4; collagen, N=3; and ristocetin, N=1). They consistently showed impaired response to ADP on FCA assay. Three patients showed impaired collagen-induced platelet aggregation on FCA assay, while one patient showed a normal response, which can be attributed to the intact b1-mediated collagen-induced aggregation in inpatients. Higher collagen concentrations led to aIIbb3-independent a2b1-activation and collagen-induced platelet aggregation in GT patients [
11,
12]. Platelet aggregates formed in GT patients were not large enough to reduce the transmission of light in LTA [
13]. The higher concentration of collagen used in FCA and the inherent sensitivity of FCA to detect even smaller aggregates of platelets enabled the detection of collagen-induced aggregation in these patients. A novel feature of FCA reported previously [
12] might be applicable in patients with a GT-like pattern on LTA and FCA assay who had normal levels of CD42b, CD41a, and CD61 on flow cytometry. On FCA assay, this patient had a marked reduction in response to collagen and an impaired response to ristocetin, which were distinct from all other three patients with a GT-like LTA pattern. A patient with a severe variant of leukocyte adhesion defect (LAD type III) can have a Glanzmann-like functional defect with a similar LTA pattern [
14]. GT patients with intact and functional ab2b1 integrin has a better response to collagen compared with LAD type III patients and hence less severe bleeding [
12]. Analysis of
ITGA2B and
ITGB3 genes or
FERMT3 mutation, which causes abnormal
kindlin-3 expression in hematopoietic cells, including platelets, confirmed the diagnosis in this patient. Although the pattern of agonist response in LTA and FCA differed with that of GT patients, the combined use of both techniques can shed light on the mechanisms underlying the pathogenesis of new diseases and differentiate the defects with a GT-like LTA pattern.
Two patients showed normal results on LTA, but exhibited an impaired response on FCA assay. The first patient had an isolated impaired response to ADP on FCA assay (13.3%), but showed a normal response to ADP on LTA. There are a few possible explanations for this finding. ADP-induced aggregation and disaggregation patterns are well described and are possibly due to the inactivation of a
IIbb
3 integrin or mechanistic reasons that lead to disassembly of the formed platelet aggregates [
15]. A significant difference was observed between the maximal aggregation on LTA and the final aggregation, and ADP-induced disaggregation was reported in all patients with a history of antiplatelet therapy (100%) [
16]. The second patient showed a borderline impaired response to ristocetin on FCA assay (16.4%) but with normal responses to ADP and collagen. The LTA data from 497 patients suggested that a single agonist abnormality should always be repeated. However, up to 22% of patients can have a persistent aggregation abnormality that is not categorized as a clear-cut platelet disorder even after repeat testing [
17]. FCA assay might have identified a defect that was not detected on LTA in these bleeding patients with a high BAT score; however, a repeat evaluation would be of additional value.
The response to ristocetin on LTA and FCA assay showed a slight statistical agreement. Five patients had an impaired response to ristocetin on LTA [4 patients with a GT-like pattern and onr patient with a borderline impaired response (65%)], and one patient had an abnormal result on FCA assay. The percentage aggregation in LTA was never markedly reduced in patients who had an impaired ristocetin response (31–65%), indicating some level of platelet agglutination to ristocetin detectable by LTA. FCA assay is a sensitive technique able to detect smaller platelet aggregates and minor platelet activation in patients with GT [
5]. This might have contributed to the normalized response to ristocetin on FCA assay in these patients and may pose a challenge in the diagnosis of Bernard-Soulier syndrome and von Willebrand disease (VWD). This may be resolved by performing a platelet glycoprotein analysis, and other tests for VWD and lower concentrations of ristocetin may be attempted.
FCA assay requires a low starting volume of PRP (200 mL for 3 agonists versus 1,500 mL in LTA). This is particularly important when evaluating pediatric patients and patients with high hematocrit and low plasma levels. FCA assay had good reproducibility and repeatability with a better CV% (1%) compared with that of LTA (7%). Our results correlate well with those of another study employing a similar technique, wherein repeated measurements of stimulated platelet mix showed a low CV% of ≤3% [
7]. FCA assay has potential applications in patients with thrombocytopenia. Collagen-induced platelet aggregation defect was identified in two ITP patients with FCA who had severe thrombocytopenia and bleeding that could otherwise have been undetected. The presence of anti-GP VI antibody in ITP patients might contribute to the development of isolated collagen-induced aggregation defects in such patients [
18]. FCA assay might help improve our understanding on the pathophysiology of ITP and other related disorders, thus aiding in the application of individualized therapeutic approaches.
This study has several limitations. The spectrum of PFDs is restricted due to the rare occurrence of PFDs and limited study duration. The steps involved in the procedure may cause platelet activation; however, they were insignificant and did not affect the final interpretation of results [
5]. This was proven in our study, where the processed sample at t=0 min (just before the addition of the agonist) showed no evidence of major platelet aggregation on FCA assay (<2%), which was confirmed by light microscopic examination. The percentage aggregation indicated by the formation of double-colored events (≥2 platelet-platelet aggregates) underestimates the true number as it excludes any small aggregates of similar color events occurring by chance [
5]. The observed range of percentage aggregation indicated by the double-colored events was relatively low; however, it is analogous to that obtained using previously established techniques [
5,
6]. The range of percentage aggregation is higher when the test is modified by mixing one portion of the labeled fraction of the sample in excess, which is useful for evaluating thrombocytopenic individuals [
7]. FCA assay quantifies platelet aggregates with estimation of average-event aggregate size; however, it does not provide information about the change in the shape of platelets, the formation of a secondary wave of platelet aggregation, or the disaggregation of platelets. The response to ADP, collagen, and ristocetin was tested using FCA assay. Epinephrine and arachidonic acid could have been used additionally, which are frequently impaired in patients with undiagnosed bleeding [
19].
The strength of this study lies in the head-to-head comparison of the LTA and FCA assay results for evaluating platelet aggregation in bleeding patients suspected to have a functional platelet defect. The FCA assay has the potential to identify PFDs in a small number of patient samples. It was able to identify PFDs in all patients diagnosed with functional platelet deficiency on LTA. The FCA assay identified two patients with reduced platelet aggregation that was not detected on LTA. The FCA assay could help understand the pathogenesis and mechanisms of these disorders. Its application in thrombocytopenic individuals would facilitate the study of platelet function in such patients. Bleeding risk assessment using FCA assay in thrombocytopenic patients might help in the selection of appropriate therapy and risk stratification. Further research including a larger cohort with a wide spectrum of PFDs and a wider panel of agonists will help cement its use in clinical settings.








 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download