1. Moris D, Ronnekleiv-Kelly S, Kostakis ID, Tsilimigras DI, Beal EW, Papalampros A, et al. 2018; Operative results and oncologic outcomes of associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) versus two-stage hepatectomy (TSH) in patients with unresectable colorectal liver metastases: a systematic review and meta-analysis. World J Surg. 42:806–815. DOI:
10.1007/s00268-017-4181-6. PMID:
28798996.

2. Donadon M, Cescon M, Cucchetti A, Cimino M, Costa G, Pesi B, et al. 2018; Parenchymal-sparing surgery for the surgical treatment of multiple colorectal liver metastases is a safer approach than major hepatectomy not impairing patients' prognosis: a bi-institutional propensity score-matched analysis. Dig Surg. 35:342–349. DOI:
10.1159/000479336. PMID:
29032372.

4. Shibata T, Niinobu T, Ogata N. 2000; Comparison of the effects of in-vivo thermal ablation of pig liver by microwave and radiofrequency coagulation. J Hepatobiliary Pancreat Surg. 7:592–598. DOI:
10.1007/s005340070009. PMID:
11180892.

5. White RR, Avital I, Sofocleous CT, Brown KT, Brody LA, Covey A, et al. 2007; Rates and patterns of recurrence for percutaneous radiofrequency ablation and open wedge resection for solitary colorectal liver metastasis. J Gastrointest Surg. 11:256–263. DOI:
10.1007/s11605-007-0100-8. PMID:
17458595.

6. Lee WS, Yun SH, Chun HK, Lee WY, Kim SJ, Choi SH, et al. 2008; Clinical outcomes of hepatic resection and radiofrequency ablation in patients with solitary colorectal liver metastasis. J Clin Gastroenterol. 42:945–949. DOI:
10.1097/MCG.0b013e318064e752. PMID:
18438208.

7. Oshowo A, Gillams A, Harrison E, Lees WR, Taylor I. 2003; Comparison of resection and radiofrequency ablation for treatment of solitary colorectal liver metastases. Br J Surg. 90:1240–1243. DOI:
10.1002/bjs.4264. PMID:
14515293.

8. Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. 2013; Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 14:1208–1215. DOI:
10.1016/S1470-2045(13)70447-9.

9. Belghiti J, Noun R, Zante E, Ballet T, Sauvanet A. 1996; Portal triad clamping or hepatic vascular exclusion for major liver resection. A controlled study. Ann Surg. 224:155–161. DOI:
10.1097/00000658-199608000-00007. PMID:
8757378. PMCID:
PMC1235336.
10. Brauer DG, Nywening TM, Jaques DP, Doyle MB, Chapman WC, Fields RC, et al. 2016; Operative site drainage after hepatectomy: a propensity score matched analysis using the American College of Surgeons NSQIP targeted hepatectomy database. J Am Coll Surg. 223:774–783.e2. DOI:
10.1016/j.jamcollsurg.2016.09.004. PMID:
27793459. PMCID:
PMC5125822.

11. Dindo D, Demartines N, Clavien PA. 2004; Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 240:205–213. DOI:
10.1097/01.sla.0000133083.54934.ae. PMID:
15273542. PMCID:
PMC1360123.
12. Rajaganeshan R, Prasad R, Guillou PJ, Chalmers CR, Scott N, Sarkar R, et al. 2007; The influence of invasive growth pattern and microvessel density on prognosis in colorectal cancer and colorectal liver metastases. Br J Cancer. 96:1112–1117. DOI:
10.1038/sj.bjc.6603677. PMID:
17353920. PMCID:
PMC2360131.

13. de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Schulick RD, et al. 2009; Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 250:440–448. DOI:
10.1097/SLA.0b013e3181b4539b. PMID:
19730175.
14. Bai H, Huangz X, Jing L, Zeng Q, Han L. 2015; The effect of radiofrequency ablation vs. liver resection on survival outcome of colorectal liver metastases (CRLM): a meta-analysis. Hepatogastroenterology. 62:373–377.
15. Ng KM, Chua TC, Saxena A, Zhao J, Chu F, Morris DL. 2012; Two decades of experience with hepatic cryotherapy for advanced colorectal metastases. Ann Surg Oncol. 19:1276–1283. DOI:
10.1245/s10434-011-2025-4. PMID:
21913018.

16. Takahashi H, Kahramangil B, Kose E, Berber E. 2018; A comparison of microwave thermosphere versus radiofrequency thermal ablation in the treatment of colorectal liver metastases. HPB (Oxford). 20:1157–1162. DOI:
10.1016/j.hpb.2018.05.012. PMID:
29929785.

17. Shady W, Petre EN, Do KG, Gonen M, Yarmohammadi H, Brown KT, et al. 2018; Percutaneous microwave versus radiofrequency ablation of colorectal liver metastases: ablation with clear margins (A0) provides the best local tumor control. J Vasc Interv Radiol. 29:268–275.e1. DOI:
10.1016/j.jvir.2017.08.021. PMID:
29203394. PMCID:
PMC5803367.
18. Puijk RS, Ruarus AH, Vroomen LGPH, van Tilborg AAJM, Scheffer HJ, Nielsen K, et al. 2018; Colorectal liver metastases: surgery versus thermal ablation (COLLISION)- a phase III single-blind prospective randomized controlled trial. BMC Cancer. 18:821. DOI:
10.1186/s12885-018-4716-8. PMID:
30111304. PMCID:
PMC6094448.

19. Wagner JS, Adson MA, Van Heerden JA, Adson MH, Ilstrup DM. 1984; The natural history of hepatic metastases from colorectal cancer. A comparison with resective treatment. Ann Surg. 199:502–508. DOI:
10.1097/00000658-198405000-00002. PMID:
6721600. PMCID:
PMC1353475.
20. Goffredo P, Utria AF, Beck AC, Chun YS, Howe JR, Weigel RJ, et al. 2019; The prognostic impact of KRAS mutation in patients having curative resection of synchronous colorectal liver metastases. J Gastrointest Surg. 23:1957–1963. DOI:
10.1007/s11605-018-3978-4. PMID:
30276588.

21. Creasy JM, Sadot E, Koerkamp BG, Chou JF, Gonen M, Kemeny NE, et al. 2018; The impact of primary tumor location on long-term survival in patients undergoing hepatic resection for metastatic colon cancer. Ann Surg Oncol. 25:431–438. DOI:
10.1245/s10434-017-6264-x. PMID:
29181680. PMCID:
PMC7480216.

22. Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. 2016; ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 27:1386–1422. DOI:
10.1093/annonc/mdw235. PMID:
27380959.

23. Acciuffi S, Meyer F, Bauschke A, Settmacher U, Lippert H, Croner R, et al. 2018; Analysis of prognostic factors after resection of solitary liver metastasis in colorectal cancer: a 22-year bicentre study. J Cancer Res Clin Oncol. 144:593–599. DOI:
10.1007/s00432-018-2583-y. PMID:
29340767.

24. Hosokawa I, Allard MA, Mirza DF, Kaiser G, Barroso E, Lapointe R, et al. 2017; Outcomes of parenchyma-preserving hepatectomy and right hepatectomy for solitary small colorectal liver metastasis: a LiverMetSurvey study. Surgery. 162:223–232. DOI:
10.1016/j.surg.2017.02.012. PMID:
28434557.

25. Shin H, Kim CW, Lee JL, Yoon YS, Park IJ, Lim SB, et al. 2019; Solitary colorectal liver metastasis after curative intent surgery: prognostic factors affecting outcomes and survival. ANZ J Surg. 89:61–67. DOI:
10.1111/ans.14933. PMID:
30484933.

26. Ou S, Xu R, Li K, Chen Y, Kong Y, Liu H, et al. 2018; Radiofrequency ablation with systemic chemotherapy in the treatment of colorectal cancer liver metastasis: a 10-year single-center study. Cancer Manag Res. 10:5227–5237. DOI:
10.2147/CMAR.S170160. PMID:
30464620. PMCID:
PMC6217171.

27. Pathak S, Palkhi E, Dave R, White A, Pandanaboyana S, Prasad KR, et al. 2016; Relationship between primary colorectal tumour and location of colorectal liver metastases. ANZ J Surg. 86:408–410. DOI:
10.1111/ans.12767. PMID:
25040656.

28. Shady W, Petre EN, Vakiani E, Ziv E, Gonen M, Brown KT, et al. 2017; Kras mutation is a marker of worse oncologic outcomes after percutaneous radiofrequency ablation of colorectal liver metastases. Oncotarget. 8:66117–66127. DOI:
10.18632/oncotarget.19806. PMID:
29029497. PMCID:
PMC5630397.

29. Deshwar A, Margonis GA, Andreatos N, Barbon C, Wang J, Buettner S, et al. 2018; Double KRAS and BRAF mutations in surgically treated colorectal cancer liver metastases: an international, multi-institutional case series. Anticancer Res. 38:2891–2895. DOI:
10.21873/anticanres.12535.

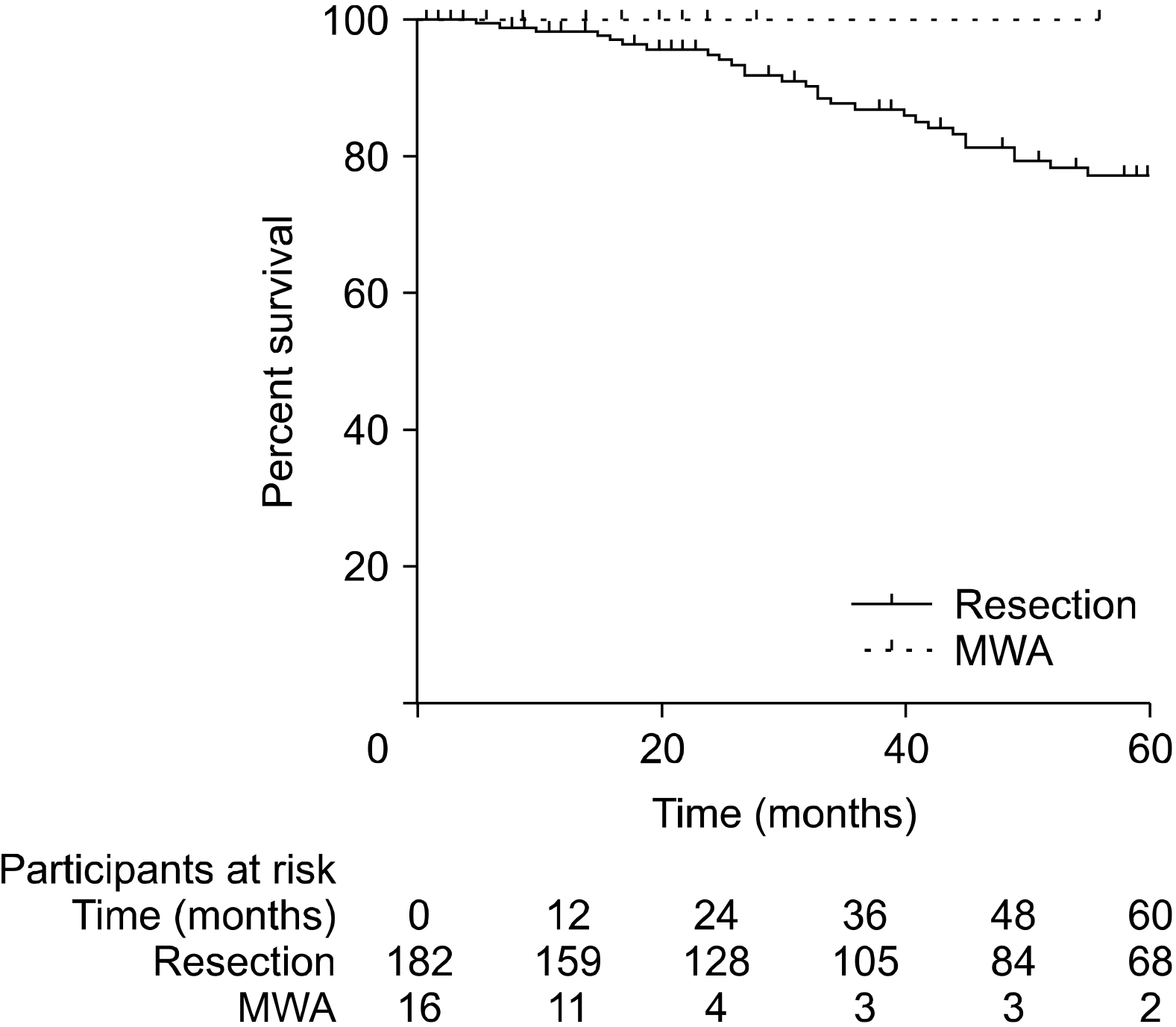
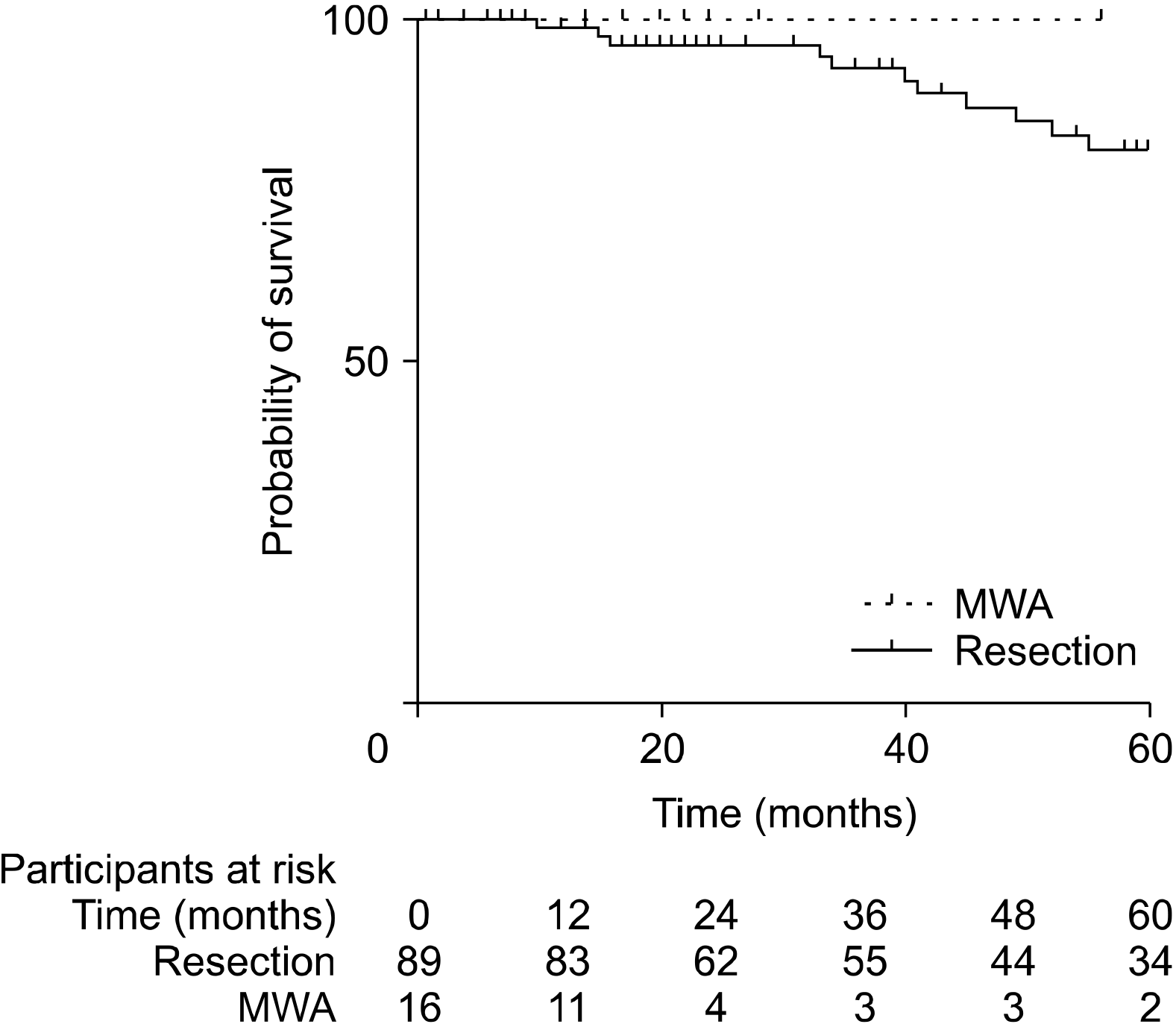
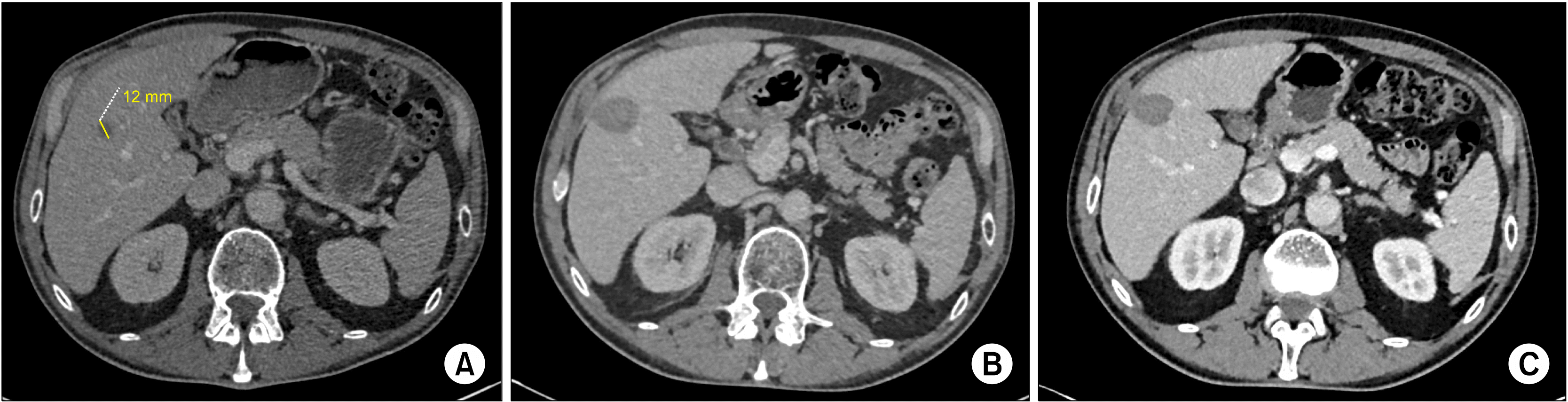




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download