Abstract
Background and Objectives
The aim of this study was to determine the clinical effectiveness of parotid gland (PG) massage for the prevention of PG dysfunction after administration of radioiodine (I-131) therapy for treatment of differentiated thyroid cancer (DTC).
Materials and Methods
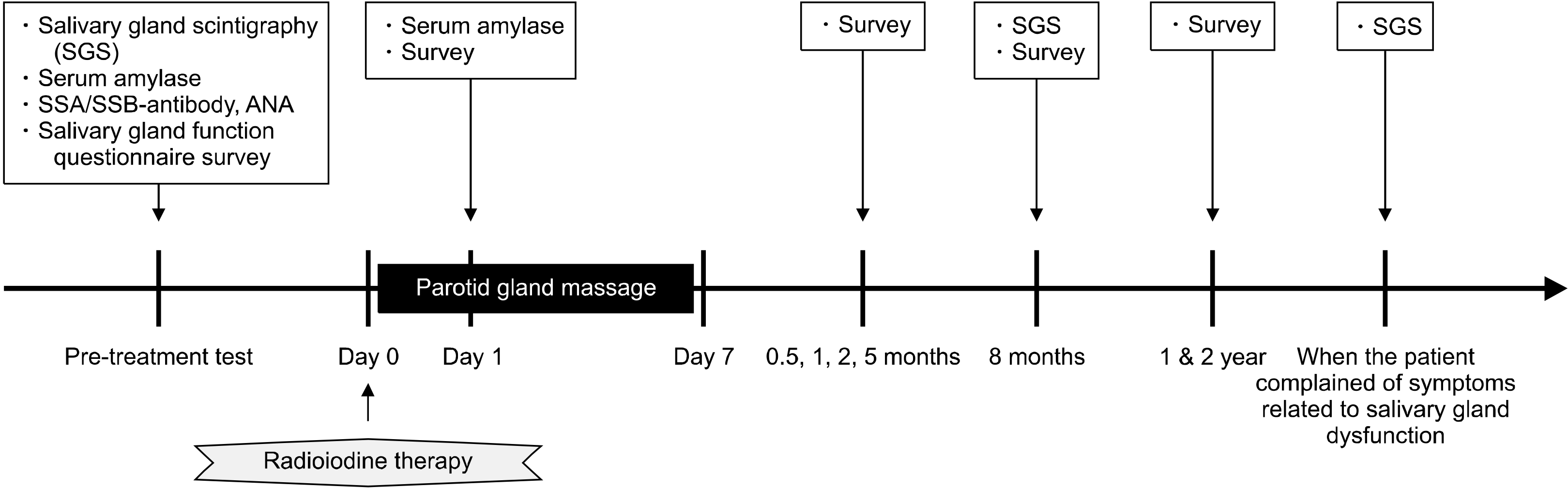
One hundred patients with DTC with planned high-dose I-131 therapy were enrolled in the clinical trial and randomized into two groups (massage and non-massage group). Serum amylase values were obtained before and 24 h after I-131 therapy, and salivary gland scintigraphy (SGS) were taken before and at eight months after the I-131 therapy. Additional SGS (addSGS) were taken when the patients complained symptoms related to salivary gland dysfunction. Questionnaire surveys were performed before and until two years after I-131 therapy.
Results
Ninety-five of 100 patients finished the study protocol. Changes in survey scores tended to be higher in the non-massage group. The non-massage group had more severe symptoms related to salivary gland dysfunction. Among 32 patients who underwent addSGS, 27 had normal 8-month SGS. Of these 27 patients, 18 (66.7%) had salivary gland dysfunction on the addSGS. Amylase values were significantly increased in patients with normal 8-month SGS but abnormal addSGS, as compared to patients who were normal on both 8-month SGS and addSGS (p=0.046). Amylase difference values were a significant predictor of abnormal addSGS (p=0.002).
Go to : 
Radioiodine (I-131) is widely used for the ablation of remnant thyroid tissue and treatment of unresectable thyroid cancer.1-4) One of the most frequent side effects of I-131 therapy is a radiation-induced sialadenitis secondary to radiation from the uptake of I-131 into the salivary glands. About 33-50% of patients who undergo I-131 therapy suffer acute uncomfortable symptoms, and 12-43% of patients may experience a slower onset of symptoms related to chronic radiation sialadenitis, which may persist for life.5-7) External massage of the parotid gland (PG) is proposed as an additional method for alleviating salivary gland injury caused by I-131 therapy, and the clinical effectiveness of PG massage was confirmed by previous prospective randomized studies.8-10) Researchers substantiated that PG massage is effective in reducing the radiation burden of the PG after administration of I-131; thus, PG massage could help prevent PG dysfunction after I-131 therapy. In a recent study,10) PG massage was preliminarily investigated but assessment of the results continued only until the 8-month follow-up. This study was performed to assess the long-term clinical effectiveness of PG massage during I-131 therapy for the prevention of PG dysfunction over a longer follow-up period, as an extension of the previous study.
Go to : 
This study was the extended version of the previous study,10) therefore the majority of the protocol was similar to it.
From October 2013 to July 2017, 100 patients were recruited and consecutively enrolled in accordance with inclusion and exclusion criteria.
The inclusion criteria of this study were as follows: (1) at least 18 years of age, (2) a recent history of total or subtotal thyroidectomy for differentiated thyroid cancer (DTC), and (3) a plan for I-131 therapy to ablate the remnant thyroid tissue.
The exclusion criteria of this study were the following: (1) distant cancer metastasis, (2) a history of external radiation exposure to the head and neck, or I-131 therapy, (3) salivary gland disorders (e.g., Sjögren syndrome), (4) diabetes, (5) collagen tissue disease (e.g., rheumatoid arthritis), or (6) diseases related to kidney function (e.g., chronic kidney disease).
The study was approved by the Institutional Review Board, and all subjects signed an informed consent form prior to participation in the study.
The study was a parallel-group, randomized clinical trial (CRIS, Clinical Research Information Service; KCT0003276; https://cris.nih.go.kr) with two arms allocated in a 1:1 ratio. One hundred patients were divided into two groups (non-massage group vs. massage group) by means of a random number table. All patients were treated with 2.96-7.4 GBq of I-131, regardless of treatment group.
The following protocols were used as methods to protect salivary glands from damage caused by radioiodine therapy. For both the non-massage and massage groups, the patients ingested sialogogues (e.g., lemon juice, candy, and chewing gum) every 30 minutes for 4 hours after I-131 administration, with at least 2 L of water consumption a day. A PG massage was performed for one minute immediately after sialogogue ingestion for patients in the massage group. These protocols were performed at waking time for 7 days after I-131 administration. The performance record for the protocols was distributed to the patients and indicated to be filled in to improve adherence to the treatment protocol. Moreover, in order to improve performance, the recommendation was made over the phone during the period after discharge.
After receiving training for PG massage from a skilled physician, the patients in the massage group performed the massage by themselves. Bilateral PGs were simultaneously massaged from the posterior to the anterior direction along the parotid duct using both palms. During the PG massage, the patient was required to clench their teeth to temporarily fix the PG in place by tightening the masticator muscles. The PG massage was performed 20 times within one minute.
The present study design and protocol are shown in Fig. 1, which is based on 8-month assessment data from a previous study.10)
Additional salivary gland scintigraphy (addSGS) was performed after the 8-month SGS if the patient complained of newly developed or aggravated symptoms related to salivary gland dysfunction. Questionnaire surveys were performed at one and two years after I-131 therapy.
The addSGS was performed as previously described.10) The decision to consider an abnormality was based on visual assessment. All the studies were independently reviewed by two experienced nuclear medicine physicians while blinded to the original clinical reports and clinical information. Significant decrease in salivary gland uptake among the baseline and follow-up SGSs was defined to be abnormal. In addition, a significant decrease (>10%) in ejection fraction (EF) in the parotid or submandibular gland (SMG) was also considered abnormal according to the previous studies. Any abnormalities of the PG and SMG were compared between the 8-month SGS and the addSGS.
Serum salivary type amylase isoenzyme was measured as an indicator of salivary gland damage. This was based on the previous studies which suggested that the changes in serum amylase level can serve as a sensitive and quantitative measure of radiation damage in the serous part of the salivary gland tissue.11-13) The amylase difference value was calculated by the difference between the pre- and post-I-131 therapy serum amylase values and was used for a Kaplan-Meier survival analysis. The amylase difference value was compared between patients with normal addSGS and those patients with abnormal addSGS to determine the effect of salivary gland damage from I-131 therapy.
Table 1 shows the sample of the salivary function questionnaire survey. Questionnaire surveys were performed before I-131 therapy (0 day), and at 2 days, 2 weeks, 1 month, 2 months, 5 months, 8 months, 1 year, and 2 years after I-131 therapy. For clarification, the questionnaire was classified into 2 parts, as the questionnaire not related to xerostomia which contained 5 questions, or as the questionnaire related to xerostomia which contained 8 questions.14) The questionnaire about symptoms not related to xerostomia, contained 4 questions with 11-point scales for assessing the severity of salivary gland swelling, pain on the salivary glands, salivary gland periductal pressure, change in taste, and 1 question about the presence of tear overflow (epiphora). The questionnaire about symptoms related to xerostomia, contained 8 questions with 11-point scales to assess the severity of difficulty to talk, chew, swallow solid food, sleep, eat because of xerostomia, severity of xerostomia while eating, and drinking water or beverages while in the eating or resting state. Higher scores indicate more severe symptoms.
The questionnaire for the salivary gland function
To compare the score of questionnaire survey between massage and non-massage groups, the change of score from 0 day was calculated in each patient, in each time point (2 days, 2 weeks, 1 month, 2 months, 5 months, 8 months, 1 year, and 2 years). Then the mean value of score change of all patients were calculated in each time point, and compared between massage and non-massage groups.
Data are expressed as means±standard deviation (SD). The independent samples t test or Welch test was used to compare the difference between the means of numerical data. To analyze the relationship between one dependent variable and one or more independent variables, logistic regression analysis was performed. To test the relationship between the two classification systems, the chi-square or Fisher’s exact test was performed. Four outlier data of post-therapy serum amylase were statistically detected and excluded from the related analyses.
Statistical analyses were performed using the MedCalc version 19.1.5 statistical package (MedCalc Software bvba, Ostend, Belgium). Statistical significance was accepted for values of <0.05.
Go to : 
Among 100 patients, five patients were excluded from the study. One patient, randomized to the non-massage group, dropped out of the study because the patient was scheduled to receive external radiotherapy for disease recurrence (finished the study assessments prior to the 5 month follow-up), and another patient randomized to the massage group, dropped out of the study because the baseline SGS result was abnormal (finished assessments prior to the 2 week follow-up). Two other patients randomized to the non-massage group withdrew their consent to participate in the study (finished the assessments prior to the 1 year follow-up), and the last patient, randomized to the massage group, also withdrew consent to participate in the study (finished the assessments prior to 1 month follow-up). Ninety-five patients remained and completed the study. Of the 95 patients, two patients refused blood sampling for serum amylase concentration 24 hours after I-131 therapy, and three patients refused the 8-month SGS. None of the patients had any other missing data for the study.
The patients’ demographics are summarized in Table 2, showing no significant between-group differences, except for the methods of preparation for the I-131 therapy (p=0.024). Most of the patients received I-131 therapy using recombinant human thyroid stimulating hormone (rhTSH), while the PG massage group had more patients treated with the thyroid hormone withdrawal (THW) method than the non-massage group.
Patients’ demographic data
Results of all surveys until the 2-year follow-up were analyzed. In 81 (90.5%) of 95 patients, the score of salivary gland dysfunction or xerostomia was increased after I-131 therapy.
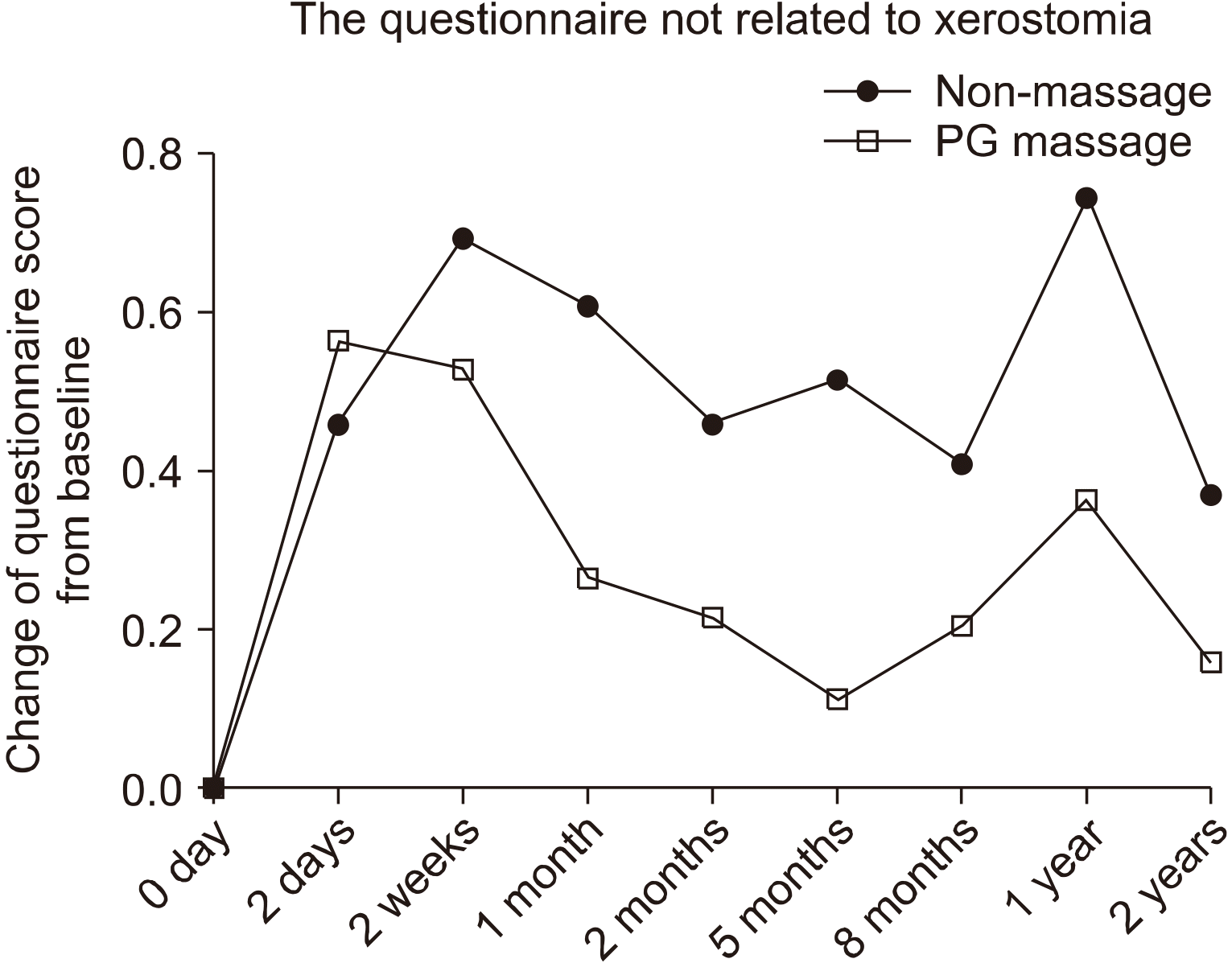
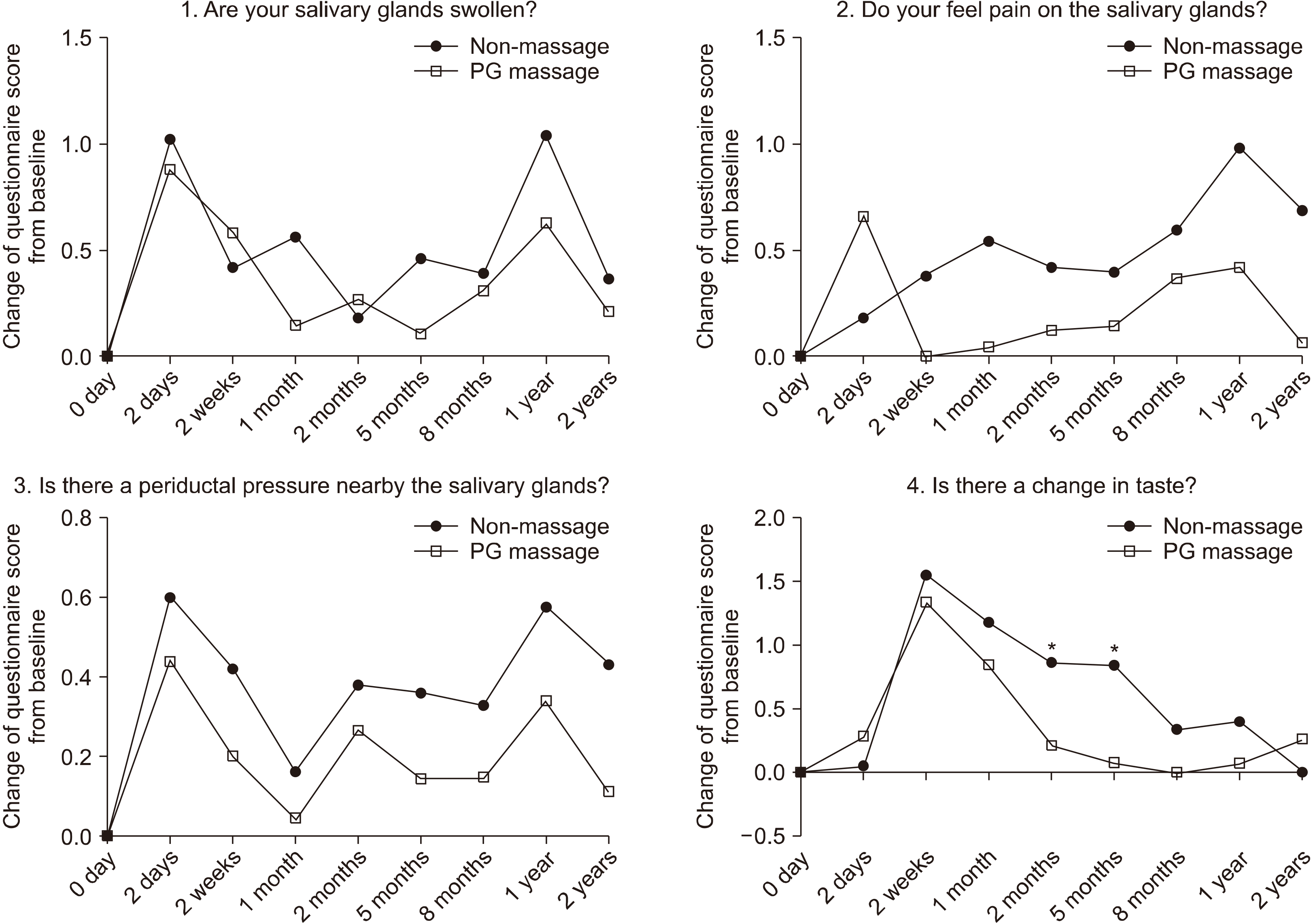
For the further statistical analyses, the score change was calculated by the difference between the pre- (0 day) and post-I-131 therapy (2 days, 2 weeks, 1 month, 2 months, 5 months, 8 months, 1 year, and 2 years) scores. For the questionnaire not related to xerostomia, although it was not statistically significant, the mean values of the score changes were higher with the patients in the non-massage group, except on day 2 (Fig. 2). Each item of the survey questionnaire was analyzed separately. On question 1, except at 2 weeks and 2 months, all values of the score change were lower for the massage group. On question 2, except at 2 days, all values were lower for patients in the massage group. On question 3, all values were lower in the massage group. On question 4, except at 2 days and 2 years, all values were lower in the massage group. The mean values of score changes were significantly higher with the patients in the non-massage group only on question 4 (Fig. 3).
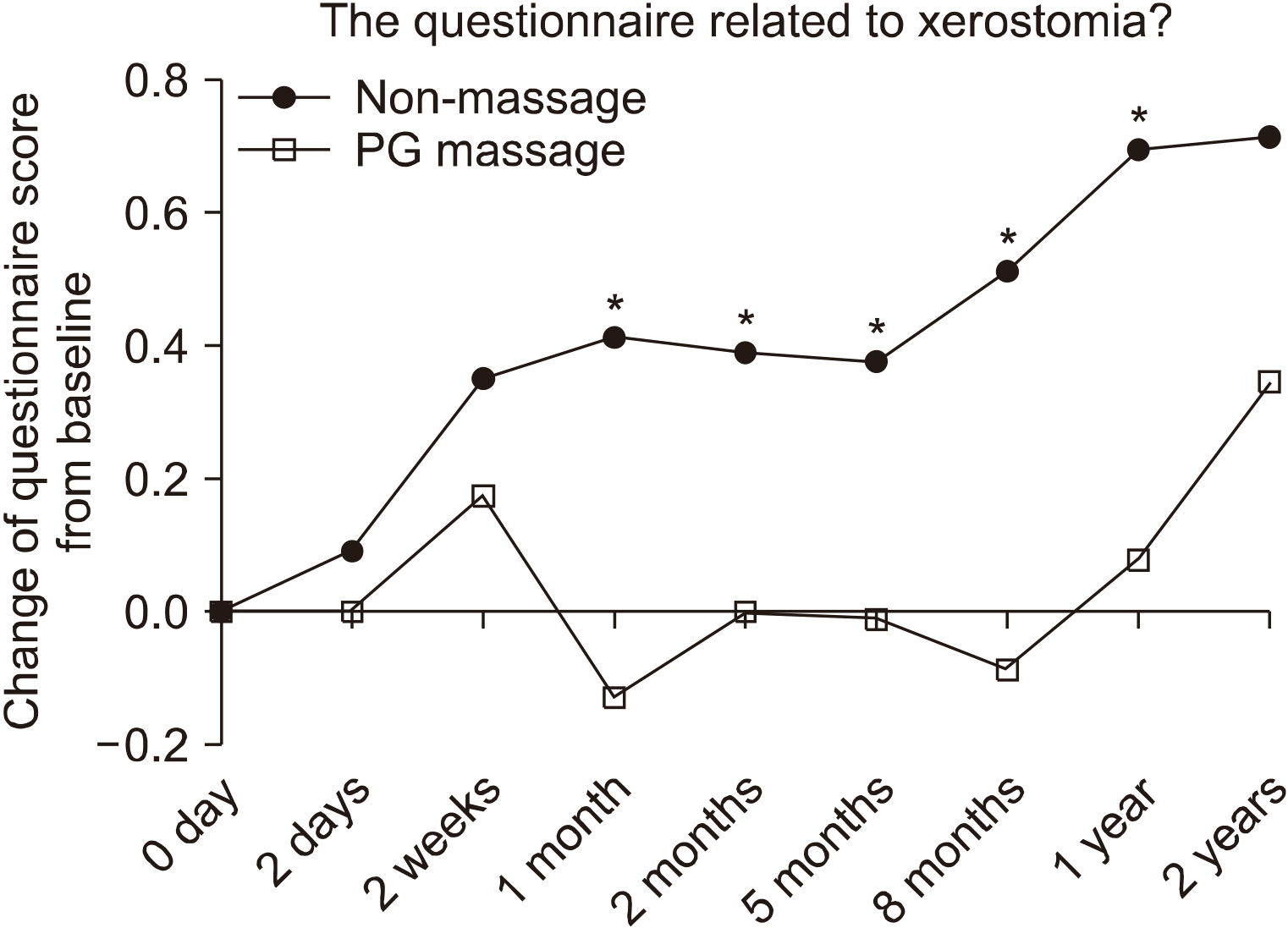
For the questionnaire related to xerostomia, the mean values of the score changes were significantly higher in the non-massage group after 1 month of follow-up, except at 2 years (Fig. 4). Each item of the survey questionnaire was analyzed in the same manner as the questionnaire not related to xerostomia. On question 1, 3, 6, and 8, all values of score change were lower in the massage group. On question 2, except at 2 months, all values were lower in the massage group. On question 4 and 7, except at 2 days, all values were lower in the massage group. On question 5, except at 2 weeks and 2 months, all values were lower in the massage group. The mean values of score changes were significantly higher with the patients in the non-massage group only on question 1, 4, and 6 (Fig. 5).
Thirty-three patients underwent addSGS. One of 33 patients denied performance of the 8-month SGS, therefore, there was no data to compare with the addSGS test. This patient was excluded from the analysis. As a result, there were sixteen (50%) patients in both non-massage and massage groups. The time period from the 8-month SGS to addSGS for the non-massage and massage groups was 28.9 and 31.1 months, respectively (p=0.6909). Among the 32 total patients, five patients had abnormal salivary gland function on the 8-month SGS, and the other 27 patients had normal salivary function. The SGS abnormality on the addSGS was more common in the non-massage group than in the massage group (75.0% vs. 62.5%, respectively), although it was not statistically significant. Salivary gland dysfunction was newly observed on the addSGS in 18 of 27 (66.7%) patients, and its occurrence, although not statistically significant, was more common in the non-massage group than in the massage group (76.9% vs. 57.1%). In the patients who had normal SGS on both the 8-month and addSGS, the EF in each gland was significantly decreased between the 8-month SGS and addSGS in the non-massage group (from 53.0% to 46.7%, p=0.0042), while there was no significant change in the massage group (from 52.1% to 51.6%, p=0.8694).
The amylase difference value was significantly higher in the patients who had a normal 8-month SGS but abnormal addSGS as compared to the patients who had normal results on both the 8-month SGS and addSGS (427.5±427.3 U/L vs. 179.4±140.2 U/L; p=0.046).
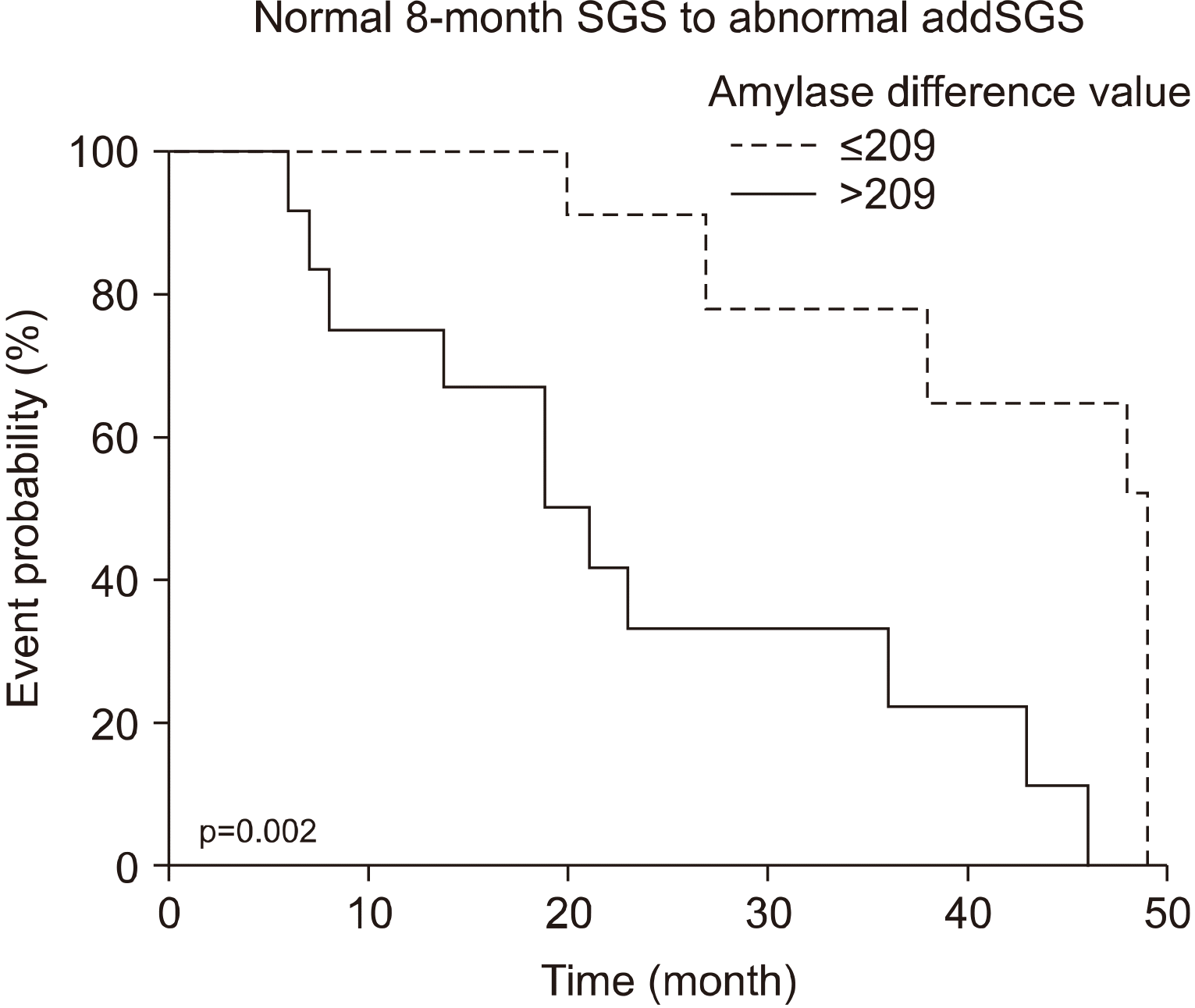
For further analysis, receiver operating characteristic (ROC) curve analysis was performed to determine the optimal cutoff values of the amylase difference value for the prediction of SGS worsening. The ROC curve demonstrated that the optimal cutoff value of amylase difference value was 209 U/L. Univariate analysis revealed that the amylase difference value (>209 U/L vs. ≤209 U/L) was a significant predictor of abnormal addSGS (p=0.002, Fig. 6).
Go to : 
This study is an extension of a previous study to demonstrate additional clues for the clinical effectiveness of PG massage for protecting salivary glands from damage caused by I-131 therapy.10) In this study, questionnaire survey data were collected until 2 years after the I-131 therapy, and results showed that the salivary gland dysfunction tended to be less salivary gland dysfunction in the massage group, and several questionnaire survey results showed statistical signifi-cance. In addition, although it was not statistically significant, the addSGS performed after the 8-month SGS showed less salivary gland dysfunction during follow-up (mean, 30 months) in the massage group. When comparing the addSGS results of 27 patients with normal 8-month SGS results, the amylase difference value for patients with abnormal addSGS was significantly higher than that of the patient group with normal addSGS. Furthermore, the risk of salivary gland dysfunction was higher in the patients who had higher amylase difference values. The study reaffirms the clinical significance of PG massage for protecting damage of salivary glands from the I-131 therapy.
In patients with DTC, I-131 therapy is a well-known and essential treatment to reduce cancer recurrence and to improve survival rates. Among the side effects that occur with I-131 therapy, salivary gland damage is irreversible in many cases and causes lifelong discomfort to patients, therefore, many investigative efforts have been applied to avoid salivary gland damage. Damage occurs when the salivary gland concentrates iodine through a sodium/iodide symporter, similar to that which happens in the thyroid tissue.15-18) A typical preventive method is the administration of sialogogues such as candies, gum, juices, and other sour foods to release stagnant I-131 from the salivary glands. It is very simple to perform, but here it was not enough to effectively prevent salivary gland damage. Therefore, the addition of PG massage to the intake of sialogogues might help prevent or reduce salivary gland damage, and this study confirmed the longer-term clinical efficacy of PG massage. However, PG massage every 30 minutes for seven days might be considered a laborious procedure for certain patients, and may not be appropriate for patients with physical handicaps. Development and application of an automatic massage machine for salivary glands may be helpful to assist patients.
Salivary glands are made up of highly differentiated cells, however, these cells are exquisitely sensitive to radiation,19) unlike classically radiosensitive tissues. Within a few days or weeks of irradiation, sialadenitis occurs due to high levels of cell death. Because I-131 is mainly concentrated in the ductal system, β-radiation may generate luminal debris, which may cause ducts to narrow.17) These processes can lead to obstruction of the ductal system, causing an inflammatory response in secretory tissues (sialadenitis), and glandular degeneration.20) Therefore, patients with sialadenitis usually complain of swelling or pain of the salivary gland, but most cases heal spontaneously.17) Incorpo-ration of damage induced by ionizing radiation into the genetic structure of the cells is assumed to result in permanent gland damage.21) Salivary gland stem cells, which are proposed to mainly reside in the excretory ducts,22) may be especially affected because of exposure to β-radiation from I-131 giving rise to reduced regenerative potential.23) These effects may lead to atrophy of the secretory parenchymal cells. Loss or impairment of serous acinar cells and replacement by connective tissue and fibrosis, may result in decreased saliva flow rates, xerostomia, oral infections, and dental caries. Among them, xerostomia is the most prominent symptom.21,24) In this study, the survey was conducted serially before I-131 therapy and up to two years after I-131 therapy, and the results demonstrated that the 90.5% of the patients suffer from salivary gland dysfunction or xerostomia. Even if there was no statistical significance, lower questionnaire score changes indicated that there was a tendency that the patients who performed PG massage had less symptoms related to sialadenitis. In addition, there was a change in taste, with a statistically significant difference at 2 and 5 months. Other findings signify a tendency of patients who performed PG massage to have less xerostomia related symptoms from the adverse effects of I-131 therapy. Moreover, the patients in the non-massage group felt it significantly more difficult to talk and sleep because of xerostomia, and they felt xerostomia more frequently when they were not eating. The results are supported by the fact that water consumption was relatively higher in the non-massage group when not eating. Other questions were about how uncomfortable patients were with xerostomia in the process of eating, such as chewing or swallowing food. Because it is possible that patients adapted to drinking more water when eating food, the statistical results of question 2, 3, and 5 might be insignificant. This is supported by the fact that the non-massage group consumed more water. Dietary adaptations are required if individuals are to maintain adequate nutrition and hydration,25,26) which have a significant impact on their quality of life.
Performance of addSGS can be an objective indicator of progression of salivary gland dysfunction.24) Patients with normal function on 8-month SGS but worsened function with addSGS were more commonly found in the non-massage group. This result was not statistically significant, presumably due to the small number of patients. If the number of patients were larger, the results would likely be significant. Among the 27 patients who received addSGS, 25 patients had amylase measured during I-131 therapy and their results were analyzed for amylase difference values. The amylase difference value was higher in the patients with abnormal addSGS. In addition, the log-rank test indicated that salivary gland dysfunction and SGS abnormality occurred earlier in patients with higher amylase difference value. Taken together, the results of PG massage after the administration of I-131 might significantly reduce the degree of salivary gland damage in the acute phase, and therefore, reduce the salivary gland dysfunction due to radiation.
According to the previous study,24) a higher prevalence of chronic salivary gland dysfunction was observed in patients with a history of acute sialadenitis. Many other studies about the adverse effects of external radiotherapy on salivary glands suggest that the chronic effects of radiation, which occur months or years after irradiation, may be the consequence of acute damage to salivary glands.27,28) Marmary et al.29) demonstrated that manifestations of cellular senescence, caused by excess DNA damage, ultimately lead to chronic salivary gland dysfunction. Senescence is a state of stable proliferative arrest that cells undergo in response to DNA-damaging agents and oxidative stress.30) This cellular senescence would lead to a breakdown in the regenerative capacity required for replacement of senescent and dysfunctional acini, thus culminating in the reduction of gland function. In addition to this fact, limited regenerative capability of the salivary glands may have caused the salivary gland dysfunction to appear late and sustained for months and years, as observed in our study. The PG massage may reduce the DNA damage to the PGs by lowering the radiation burden and consequentially decreasing cellular senescence, thus preventing chronic salivary gland dysfunction.
There are several limitations in this study. First, the PG massage method was not optimized. The PGs were massaged for the same period (one minute) for seven days. Certain performance parameters for the massage method should be optimized in future studies, including the number of times the glands are massaged, the time period, and the level of massage skill taught to the patients. Development of automatic massage machines for use with salivary glands can be one of the alternatives to standardize the manner of massage. Second, although patients were instructed to fill in the performance record chart, there were no means to actively encourage the massage such that the patients may not have been as diligent in performing the massage. However, it is not possible to forcibly recommend patients to perform the PG massage because of the ethical issues.
In conclusion, PG massage reduced the occurrence of symptoms related to salivary gland dysfunction. The PG massage may be helpful to prevent damage of salivary gland caused by I-131 therapy.
Go to : 
Acknowledgments
This research was supported by the Industry-Academy Collaborative Research in the form of an investigator-sponsored study through Kyungpook University Hospital and Sanofi-Genzyme Korea. The authors hereby attest and disclose that the study was funded by Sanofi-Genzyme Korea. However, any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of Sanofi-Genzyme Korea. The study was neither designed nor modified to reflect particular interests, and the authors whose names are listed in this study certify that they have no affiliations or involvement with Sanofi-Genzyme Korea and declare no conflicts of interests with the entity. This research was also supported by a grant of the Daegu-Gyeongbuk/Osong Medical Cluster R&D Project funded by the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, Republic of Korea (grant number: HI19C0760).
Go to : 
References
1. Ahn BC. 2016; Personalized medicine based on theranostic radioiodine molecular imaging for differentiated thyroid cancer. Biomed Res Int. 2016:1680464. DOI: 10.1155/2016/1680464. PMID: 27239470. PMCID: PMC4864569.

2. Hong CM, Ahn BC. 2017; Redifferentiation of radioiodine refractory differentiated thyroid cancer for reapplication of I-131 therapy. Front Endocrinol (Lausanne). 8:260. DOI: 10.3389/fendo.2017.00260. PMID: 29085335. PMCID: PMC5649198.

3. Hong CM, Kim CY, Son SH, Jung JH, Lee CH, Jeong JH, et al. 2017; I-131 biokinetics of remnant normal thyroid tissue and residual thyroid cancer in patients with differentiated thyroid cancer: comparison between recombinant human TSH admini-stration and thyroid hormone withdrawal. Ann Nucl Med. 31(8):582–9. DOI: 10.1007/s12149-017-1188-x. PMID: 28677070.

4. Jeong JH, Kong EJ, Jeong SY, Lee SW, Cho IH, Ah Chun K, et al. 2017; Clinical outcomes of low-dose and high-dose post-operative radioiodine therapy in patients with intermediate-risk differentiated thyroid cancer. Nucl Med Commun. 38(3):228–33. DOI: 10.1097/MNM.0000000000000636. PMID: 27984538.

5. Alexander C, Bader JB, Schaefer A, Finke C, Kirsch CM. 1998; Intermediate and long-term side effects of high-dose radioiodine therapy for thyroid carcinoma. J Nucl Med. 39(9):1551–4. PMID: 9744341.
6. Allweiss P, Braunstein GD, Katz A, Waxman A. 1984; Sialadenitis following I-131 therapy for thyroid carcinoma: concise com-munication. J Nucl Med. 25(7):755–8. PMID: 6737074.
7. Kita T, Yokoyama K, Higuchi T, Kinuya S, Taki J, Nakajima K, et al. 2004; Multifactorial analysis on the short-term side effects occurring within 96 hours after radioiodine-131 therapy for differentiated thyroid carcinoma. Ann Nucl Med. 18(4):345–9. DOI: 10.1007/BF02984474. PMID: 15359929.
8. Hong CM, Son SH, Kim CY, Kim DH, Jeong SY, Lee SW, et al. 2014; Emptying effect of massage on parotid gland radioiodine content. Nucl Med Commun. 35(11):1127–31. DOI: 10.1097/MNM.0000000000000176. PMID: 25121980.

9. Kim HW, Ahn BC, Lee SW, Lee J. 2012; Effect of parotid gland massage on parotid gland Tc-99m pertechnetate uptake. Thyroid. 22(6):611–6. DOI: 10.1089/thy.2011.0188. PMID: 22524471. PMCID: PMC3358109.

10. Son SH, Lee CH, Jung JH, Kim DH, Hong CM, Jeong JH, et al. 2019; The preventive effect of parotid gland massage on salivary gland dysfunction during high-dose radioactive iodine therapy for differentiated thyroid cancer: a randomized clinical trial. Clin Nucl Med. 44(8):625–33. DOI: 10.1097/RLU.0000000000002602. PMID: 31274608.
11. Becciolini A, Giannardi G, Cionini L, Porciani S, Fallai C, Pirtoli L. 1984; Plasma amylase activity as a biochemical indicator of radiation injury to salivary glands. Acta Radiol Oncol. 23(1):9–14. DOI: 10.3109/02841868409135978. PMID: 6203336.

12. Kishima HK, Kirkham WR, Andrews JR. 1965; Post-irradiation sialadenitis. A study of the clinical features, histopathologic changes and serum enzyme variations following irradiation of human salivary glands. Am J Roentgenol Radium Therapy Nucl Med. 94:271–91.
13. Van den Brenk HA, Hurley RA, Gomez C, Richter W. 1969; Serum amylase as a measure of salivary gland radiation damage. Hyperamylasaemia following fractionated exposure to 4 MV X rays delivered in high pressure oxygen, and effects of certain steroids on this response. Br J Radiol. 42(501):688–700. DOI: 10.1259/0007-1285-42-501-688. PMID: 4981289.
14. Eisbruch A, Kim HM, Terrell JE, Marsh LH, Dawson LA, Ship JA. 2001; Xerostomia and its predictors following parotid-sparing irradiation of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 50(3):695–704. DOI: 10.1016/S0360-3016(01)01512-7. PMID: 11395238.

15. Ahn BC. 2012; Sodium iodide symporter for nuclear molecular imaging and gene therapy: from bedside to bench and back. Theranostics. 2(4):392–402. DOI: 10.7150/thno.3722. PMID: 22539935. PMCID: PMC3337731.

16. De La Vieja A, Dohan O, Levy O, Carrasco N. 2000; Molecular analysis of the sodium/iodide symporter: impact on thyroid and extrathyroid pathophysiology. Physiol Rev. 80(3):1083–105. DOI: 10.1152/physrev.2000.80.3.1083. PMID: 10893432.
17. Mandel SJ, Mandel L. 2003; Radioactive iodine and the salivary glands. Thyroid. 13(3):265–71. DOI: 10.1089/105072503321582060. PMID: 12729475.

18. Oh JR, Ahn BC. 2012; False-positive uptake on radioiodine whole- body scintigraphy: physiologic and pathologic variants unrelated to thyroid cancer. Am J Nucl Med Mol Imaging. 2(3):362–85. PMID: 23133823. PMCID: PMC3477738.
19. Hall EJ. 2000. Radiobiology for the radiologist. 5th ed. Lippincott Williams & Wilkins;Philadelphia:
20. Van Nostrand D. 2011; Sialoadenitis secondary to (1)(3)(1)I therapy for well-differentiated thyroid cancer. Oral Dis. 17(2):154–61. DOI: 10.1111/j.1601-0825.2010.01726.x. PMID: 21029259.

21. Fox PC. 1998; Acquired salivary dysfunction. Drugs and radiation. Ann N Y Acad Sci. 842:132–7. DOI: 10.1111/j.1749-6632.1998.tb09641.x. PMID: 9599303.

22. van Luijk P, Pringle S, Deasy JO, Moiseenko VV, Faber H, Hovan A, et al. 2015; Sparing the region of the salivary gland con-taining stem cells preserves saliva production after radiotherapy for head and neck cancer. Sci Transl Med. 7(305):305ra147. DOI: 10.1126/scitranslmed.aac4441. PMID: 26378247. PMCID: PMC4964284.

23. Klein Hesselink EN, Links TP. 2015; Radioiodine treatment and thyroid hormone suppression therapy for differentiated thyroid carcinoma: adverse effects support the trend toward less aggressive treatment for low-risk patients. Eur Thyroid J. 4(2):82–92. DOI: 10.1159/000432397. PMID: 26279993. PMCID: PMC4521066.

24. Jeong SY, Kim HW, Lee SW, Ahn BC, Lee J. 2013; Salivary gland function 5 years after radioactive iodine ablation in patients with differentiated thyroid cancer: direct comparison of pre- and postablation scintigraphies and their relation to xerostomia symptoms. Thyroid. 23(5):609–16. DOI: 10.1089/thy.2012.0106. PMID: 23153322. PMCID: PMC3643252.

25. Hancock PJ, Epstein JB, Sadler GR. 2003; Oral and dental management related to radiation therapy for head and neck cancer. J Can Dent Assoc. 69(9):585–90. PMID: 14653934.
26. Cady J. 2007; Nutritional support during radiotherapy for head and neck cancer: the role of prophylactic feeding tube placement. Clin J Oncol Nurs. 11(6):875–80. DOI: 10.1188/07.CJON.875-880. PMID: 18063546.

27. Li Y, Taylor JM, Ten Haken RK, Eisbruch A. 2007; The impact of dose on parotid salivary recovery in head and neck cancer patients treated with radiation therapy. Int J Radiat Oncol Biol Phys. 67(3):660–9. DOI: 10.1016/j.ijrobp.2006.09.021. PMID: 17141973. PMCID: PMC2001308.

28. Stephens LC, King GK, Peters LJ, Ang KK, Schultheiss TE, Jardine JH. 1986; Acute and late radiation injury in rhesus monkey parotid glands. Evidence of interphase cell death. Am J Pathol. 124(3):469–78. PMID: 3766705. PMCID: PMC1888342.
29. Marmary Y, Adar R, Gaska S, Wygoda A, Maly A, Cohen J, et al. 2016; Radiation-induced loss of salivary gland function is driven by cellular senescence and prevented by IL6 modulation. Cancer Res. 76(5):1170–80. DOI: 10.1158/0008-5472.CAN-15-1671. PMID: 26759233.

30. Pawlikowski JS, Adams PD, Nelson DM. 2013; Senescence at a glance. J Cell Sci. 126(Pt 18):4061–7. DOI: 10.1242/jcs.109728. PMID: 23970414. PMCID: PMC3772382.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download