Introduction
Hashimoto thyroiditis (HT), an autoimmune thyroid disorder, is the most common cause of acquired hypothyroidism in youth.
1) It predominantly affects individuals during the early to mid-puberty stages, and the patients with this disease usually develop a diffuse goiter without pain or tenderness.
2,3) Patients with HT exhibit various types of thyroid function-related manifestations, such as subclinical hypothyroidism, hypothyroidism, euthyroidism, or hyperthyroidism with elevated autoantibody levels.
1,2)
Patients can present with symptoms, such as neck pain and tenderness, in very rare cases.
3) Due to the rarity of painful HT, it can be misdiagnosed as other thyroid diseases, such as subacute thyroiditis (SAT) or infection. Furthermore, there is limited evidence regarding the clinical course and appropriate treatment of this condition.
3)
Therefore, here, we report a case involving a child with painful HT. A 7-year-old girl presented with a painful goiter and consequent neck swelling, which were present for 1 year, and the laboratory findings suggested a diagnosis of HT.
Go to :

Case Report
A 7-year-old girl visited the emergency room with a chief complaint of pain in the anterior region of the neck. The pain was not radiating in nature and was not aggravated by swallowing. This symptom was apparent for three days. She had a goiter for one year, although no prior evaluation had been performed. She had cold intolerance for 6months, but oral intake was tolerable, and did not have chronic fatigue, swelling, or constipation. Her developmental status was normal, and she did not show any problems with social relationship, activity, or personality changes. She did not have any medical or treatment history, including immunosuppression, frequent infections, or any recent history of upper respiratory tract infection. Her father had type 2 diabetes and dyslipidemia. However, no one in her family had a history of thyroid disorders. She did not experience symptoms such as fever or headaches. She exhibited cold intolerance without weight change. Her vital signs were as follows: pulse rate, 90 beats/min; blood pressure, 105/68 mmHg (50-75th percentile); respiratory rate, 18 times/min; and body temperature, 37.0°C. Physical examinations revealed that both her thyroid lobes were diffusely enlarged and were 5×7 cm in size, with a firm consistency and without tenderness. No definitive nodule was palpable in either thyroid gland. Her anthropometric measurements were as follows: height, 133.2 cm (1.31 height standard deviation score [SDS]); weight, 25.6 kg (−0.39 weight SDS); and body mass index, 23.04 kg/m2 (14th percentile).
Complete blood counts, electrolyte levels, glucose levels, liver and renal function test results, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) levels were normal. Her serum thyroid-stimulating hormone (TSH) level was elevated at 227.0
μIU/mL (normal: 0.41-4.69
μIU/mL), the free thyroxine (FT4) level was 0.23 ng/dL (normal: 0.8-1.7 ng/dL), and the triiodothyronine level was 0.61 ng/mL (normal: 0.71-1.61 ng/mL). Autoantibody levels were elevated as shown in
Table 1. Thyroid ultrasonography (US) revealed a diffusely enlarged thyroid gland with homogeneously mild hypoechoic changes involving the entire area of both thyroid glands with lobulated contours (
Fig. 1). Color Doppler US showed diffuse hypervascularity without hypovascular portion in the hypoechoic lesion. No cervical lymphadenopathy was detected. Oral prednisolone (Solondo
®) 0.9 mg/kg/d, levothyroxine (Synthyroxine
®) 3.9 mcg/kg/d, and ibuprofen (Brufen syrup for children
®) 18.8 mg/kg/d were prescribed as the starting medical treatment dose (
Table 1). The neck pain improved dramatically after 10 days of treatment, and the goiter size decreased gradually. At 2 weeks after the initial treatment, her serum TSH level decreased to 8.22
μIU/mL and the FT4 level was 2.33 ng/dL (
Table 1). After improvement of the clinical symptoms, we stopped prednisolone and started oral hydrocortisone (Hysone
®) 9.7 mg/m
2/d divided into two doses because the cortisol level was not sufficient. Even after the steroid and levothyroxine was tapered gradually, she did not complain of neck pain again. At 14 weeks after initial treatment, her serum TSH level was 4.45
μIU/mL and the FT4 level was 1.57 ng/dL, and the levothyroxine was stopped (
Table 1).
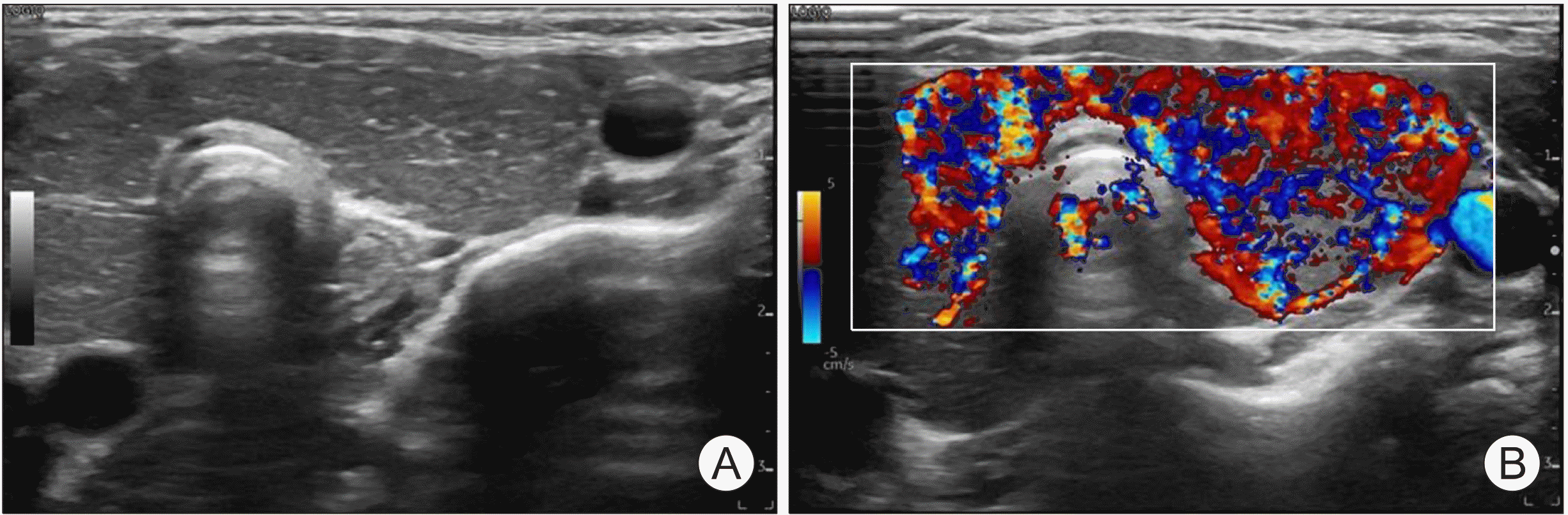 | Fig. 1Thyroid ultrasound images. (A) Ultrasonographic findings revealed a diffusely enlarged thyroid gland with homogeneously mild hypoechoic changes involving the entire area of both thyroid glands with lobulated contour. (B) Color Doppler ultrasonography showed diffuse hypervascularity without hypovascular portion in the hypoechoic lesion. 
|
Table 1
Thyroid function tests and thyroid autoantibodies in the patient
|
First visit |
2 weeks |
4 weeks |
6 weeks |
8 weeks |
10 weeks |
14 weeks |
Unit (reference range) |
|
T3 |
0.61 |
0.60 |
0.66 |
1.13 |
1.25 |
1.53 |
1.70 |
ng/mL (0.71-1.61) |
|
FT4 |
0.23 |
2.33 |
1.94 |
1.82 |
1.70 |
1.68 |
1.57 |
ng/dL (0.8-1.7) |
|
TSH |
227.0 |
8.22 |
1.30 |
2.75 |
3.83 |
2.69 |
4.45 |
μIU/mL (0.41-4.69) |
|
TPO antibody |
<9 |
|
|
|
|
|
|
IU/mL (0-34) |
|
Thyroglobulin antibody |
322.0 |
|
|
|
|
|
|
IU/mL (0-130.6) |
|
TSH receptor antibody |
<0.8 |
|
|
|
|
|
|
IU/L (0-1.75) |
|
Thyroid stimulating antibody |
198.4 |
|
|
|
|
|
|
% (<140) |
|
Prednisolone*
|
22.5 |
10 |
7.5 |
3.75 |
Stop |
|
|
mg/d |
|
Hydrocortisone*
|
|
|
|
|
10 |
10 |
10 |
mg/d |

Go to :

Discussion
Our patient exhibited anterior neck pain and a goiter as a result of HT, both of which improved dramatically following treatment with a steroid and levothyroxine. Although HT is the most common cause of acquired hypothyroidism in children, there are a very limited number of published reports regarding the clinical course and medical treatment of painful HT in children.
1,3)
HT is an autoimmune thyroid disorder induced by inflammation resulting from the infiltration of the thyroid gland by B and T cells that are reactive to thyroid antigens.
4) Patients with HT usually exhibit fatigue, weight gain, and myxoedema with a painless firm diffuse goiter. Laboratory findings often reveal hypothyroidism and the presence of autoantibodies to thyroglobulin and thyroid peroxidase. Thyroid stimulating Ab, one of TSH receptor antibodies, stimulates TSH receptor through binding to leucine rich repeat region of the extracellular domain of the TSH receptor, and is associated with the risk of HT.
5,6) US usually reveals diffusely enlarged and hypoechoic thyroid gland with a heterogeneous echotexture and echogenic septations due to lymphocyte infiltration. The histology shows reactive lymphocytic infiltrations with occasional Hürthle cell changes and a few multinucleate histiocytes; however, a cytological analysis is not mandatory to reach a diagnosis because HT can be diagnosed based on laboratory findings indicating positive thyroperoxidase antibody and thyroglobulin antibody levels accompanied by abnormal thyroid function, an enlarged thyroid gland, and morphological changes on an US of the thyroid gland.
2,7) Thus, a biopsy was not performed on our patient because her clinical signs and symptoms, laboratory findings, and US findings were indicative of HT and because of the dramatic improvement observed in her symptoms and signs as a result of medical treatment.
Although most of the patients with HT do not have neck pain, HT can be a very rare cause of thyroid pain.
2) Patients with painful HT may have clinical manifestations including fever, thyroid pain, and tenderness, with elevated levels of white blood cell count, ESR, and CRP.
2,8) However, a significant number of patients do not have the clinical manifestations of inflammation such as fever or leukocytosis. Initial thyroid function may present as hypothyroidism, euthyroidism, and hyperthyroidism. Although a literature review reported statistical analysis for disease characteristics of painful HT in adults, a case report of the clinical course of painful HT in children is very limited. Because of its rarity, painful HT can be under-recognized in children with neck pain. Thus, investigations regarding clinical manifestations and laboratory tests of HT should be considered in children with neck pain without a definite diagnosis.
Among the causes of thyroid pain, SAT and infection are more common than HT.
3) SAT is the most common reason for painful thyroiditis and is thought to be caused by viral infection.
9) Patients often develop SAT after acquiring an upper respiratory infection with fever. They complain of neck pain that radiates to the ear or jaws, which is aggravated by swallowing. A physical examination generally reveals a small diffuse goiter with tenderness. Laboratory findings reveal elevated ESR, CRP level, and leukocyte counts. On US, a thyroid gland affected by SAT has poorly defined hypoechoic avascular areas with a heterogeneous echo pattern in the acute phase, and the vascular flow increases slightly in the recovery phase.
9,10) The histology reveals intrafollicular cellular infiltration with the loss of colloid and destruction of the lining epithelium; however, a histologic examination is rarely required because SAT is usually diagnosed clinically.
2,9) Our patient exhibited neck pain; however, her past history, signs, and US findings were indicative of HT rather than SAT. Due to the rarity of painful HT, careful clinical approaches are required to differentiate painful HT from SAT in children with neck pain.
Significant numbers of patients underwent surgery due to poor response to medical treatment and high frequency of relapse in a literature review of adult painful HT.
8) Moreover, the number of thyroidectomies is increasing recently. Although oral medications cannot provide sustained pain resolution in adults generally, medical treatment with levothyroxine, steroid, and nonsteroidal anti-inflammatory drugs (NSAIDs) can be tried. In the case of hypothyroidism, levothyroxine can be effective for goiter shrinkage as well as for maintaining the euthyroid state.
11) The normalization of thyroid function has been found to occur in 16-50% of children with hypothyroidism resulting from HT. To treat the pain associated with HT in adults, oral prednisolone and NSAIDs, as well as levothyroxine, are recommended. However, a literature review of 70 cases of painful HT indicated that only 25-50% of patients experience pain relief with corticosteroid use.
8) NSAIDs failed to provide continuous pain control in most patients, and the sustained pain relief-related response rate with levothyroxine use in the absence of hypothyroidism was similar to that of oral corticosteroid use. Although studies involving the surgical treatment of painful HT in youth have been reported, a very limited number of elaborate studies involved an investigation into dosing strategies for the medication in children with painful HT.
3) Krakovitz et al.
3) reported five cases of youth with painful HT, and all of them were unable to resolve the pain with medical treatment and finally required surgical treatment. Our patient was treated with prednisolone 0.88 mg/kg/d, levothyroxine, and ibuprofen, and the pain and goiter size dramatically improved. Subsequently, the prednisolone and levothyroxine doses were gradually tapered.
In conclusion, we report a case involving a girl with painful thyroiditis that was effectively treated with medical treatment. Her neck pain improved dramatically with prednisolone, levothyroxine, and ibuprofen and did not worsen again even after the doses of the medications were tapered. Although painful HT is very rarely observed in children, it should be considered as one of the differential diagnoses in children with neck pain and goiter.
Go to :






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download