Abstract
Multisystem inflammatory disease in children is a Kawasaki disease like illness occurring after severe acute respiratory syndrome coronavirus 2 infection in children. As the pandemic progresses, similar syndromes were also reported in adult with a decreased incidence. Multisystem inflammatory syndrome in adults (MIS-A) can be characterized with shock, heart failure, and gastrointestinal symptoms with elevated inflammatory markers after coronavirus disease 2019 (COVID-19) infection. Herein, we describe the first case of MIS-A in South Korea. A 38-year-old man presented to our hospital with a 5-day history of abdominal pain and fever. He had been treated with antibiotics for 5 days at the previous hospital, but symptoms had worsened and he had developed orthopnea on the day of presentation. He suffered COVID-19 six weeks ago. Laboratory data revealed elevated white blood cell counts with neutrophil dominance, C-reactive protein, and B-type natriuretic peptide. Chest X-ray showed normal lung parenchyme and echocardiography showed severe biventricular failure with normal chamber size. We diagnosed him as MIS-A and treated with intravenous immunoglobulin and steroid.
Go to : 
Graphical Abstract
Go to : 
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can cause respiratory diseases of variable severities. In children, coronavirus disease 2019 (COVID-19) is usually known to be mild. Instead hyperinflammatory shock conditions similar to Kawasaki diseases have been reported after COVID-19,1 and later defined as a multisystem inflammatory syndrome in children (MIS-C). Clinical syndrome with similar features also have been described in adult patients.23 The MIS in adults (MIS-A) is characterized by shock with predominant extrapulmonary symptoms such as gastrointestinal, cardiovascular, dermatologic, or neurologic symptoms that occur 2–6 weeks after COVID-19. Herein, we report the first case of MIS-A in South Korea.
Go to : 
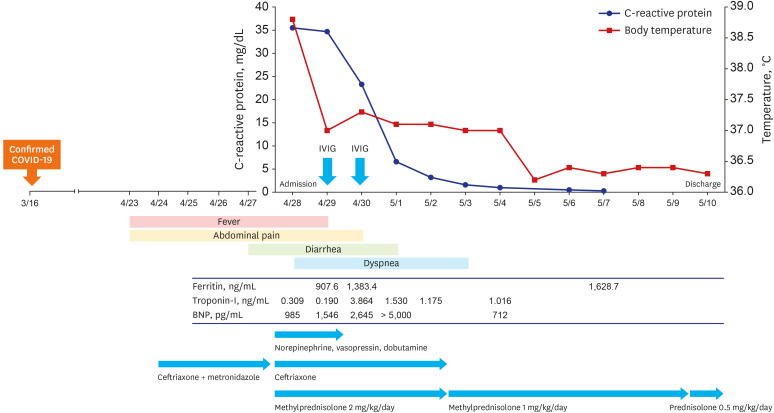
A 38-year-old man presented to our hospital with a 5-day history of fever and abdominal pain on 28th April 2021. He had been treated with intravenous ceftriaxone and metronidazole for 5 days (24–28th April) at the previous hospital. However his symptoms including abdominal pain, vomiting, and diarrhea had worsened and orthopnea newly developed on 28th April. He denied cough, sputum, or skin rash. He reported that he was diagnosed with mild COVID-19 six weeks ago (16th March). SARS-CoV-2 reverse transcription polymerase chain reaction (PCR) test obtained from nasopharyngeal swab was positive on the day of COVID-19 diagnosis and turned to negative on the day of discharge. He recovered without complications. Initial vital signs were blood pressure of 89/60 mmHg, heartbeat of 159 beats/min, respiratory rate of 30 breaths/min, and body temperature of 38.8°C. On physical examination, whole abdominal tenderness without rebound tenderness was noted and there were neither abnormal findings in lung sound nor heart sound on auscultation. Laboratory data revealed leukocytosis (white blood cells [WBC] 20,300 cells/mL) with neutrophilia (96.6%). Inflammatory markers were elevated including C-reactive protein (CRP) of 35.50 mg/dL, procalcitonin of 13.77 ng/mL, fibrinogen of 946 mg/dL, and ferritin of 907.6 ng/mL. Both the PT (15.0 sec) and activated partial thromboplastin time (44.1 sec) were prolonged. Although troponin-I was in the normal range, B-type natriuretic peptide (BNP, 985 pg/mL) was elevated. SARS-CoV-2 PCR revealed negative. SARS-CoV-2 immunoglobulin G (IgG) was tested with the LIAISON SARS-CoV-2 TrimericS IgG assay, using the indirect chemiluminescence immunoassay method. The test result was positive as 238 AU/mL (positive cut-off value, ≥ 33.8 AU/mL). Electrocardiography revealed sinus tachycardia. Chest X-ray and computed tomography (CT) showed no infiltration in the lung parenchyme. Abdominopelvic CT showed infiltration around small bowel mesentery. As being shock status, he needed high-dose norepinephrine, vasopressin, and dobutamine, and he was admitted to the intensive care unit for close monitoring.
We considered the MIS-A as the most probable diagnosis based on his history, laboratory, and image results. The blood culture was negative. Stool examination revealed no WBC on stain, and pathogenic bacteria such as Shigella, Salmonella, Yersinia, and Vibrio species did not grow in stool culture. Stool tests for Clostridioides difficile were negative by PCR and culture. The C. difficile toxin enzyme immunoassay was also negative. The negative microbiologic examination results and poor response to the antibiotics suggested that infectious condition such as septic shock with infectious gastroenteritis or the toxic shock syndrome were unlikely. We commenced treatment with methylprednisolone (mPD) 2 mg/kg/day from hospital day 1. Intravenous immunoglobulin (IVIG) 1g/kg/day were administered on hospital day 2 and 3. The patient received empirical antibiotics of ceftriaxone until the blood culture results came out negative. Fig. 1 shows the clinical courses and treatment of our patient. On hospital day 2, he became afebrile but developed new-onset chest pain with cardiac marker elevation including troponin-I of 9.81 ng/mL, creatinine kinase-MB of 44.9 ng/mL, and BNP of 1,546 pg/mL. He underwent echocardiography that revealed severe left and right ventricular dysfunction with left ventricular ejection fracture of 32%. The heart chamber size was within normal range (left ventricle inner dimensions [LVID] at end systole, 40 mm; LVID at end diastole, 50 mm). Receiving steroid and IVIG, his symptoms including orthopnea, abdominal pain, and diarrhea were improved rapidly. On hospital day 6, CRP decreased to 1.56 mg/dL and we tapered mPD to 1mg/kg/day. Echocardiography was performed for follow-up on hospital day 9 and heart function was recovered. On hospital day 13, he was discharged with tapered oral steroid, prednisolone 0.5 mg/kg/day.
Go to : 
We describe the first case of MIS-A in South Korea. Our patient showed a typical clinical course of MIS-A. With a past-history of mild COVID-19 six weeks ago, he developed fever, abdominal pain, and diarrhea, which were non-responsible with antibiotics, then orthopnea due to heart failure occurred. He had no cough or sputum and his lung on chest image was clean. He was hospitalized because of a severe shock state. After receiving steroids and IVIG, his condition rapidly improved. Clinical suspicion of MIS-A made early diagnosis result in early treatment.
Two cases of MIS-C in South Korea have been reported.45 These two patients had similar clinical findings like our patient, including fever, abdominal pain, nausea, vomiting, or diarrhea showing shock status. However, they also had conjunctival injection, strawberry tongue, or skin rash which were not developed in our patient. In the recent cross-sectional study of MIS-C,6 71.3% of MIS-C were Hispanic or non-Hispanic Black. Gastrointestinal symptoms including abdominal pain, vomiting, and diarrhea were the most common symptoms of MIS-C. Skin rash was also common observed in 55.6%. However, cardiac dysfunction was reported in 31.0%. Although data on MIS-A was limited, it seemed to be that heart involvement was more common in MIS-A than MIS-C and skin rash was more common in MIS-C than MIS-A. Gastrointestinal tract was the most frequently involved organ system in both MIS-A and MIS-C.7 In previous case series of MIS-A,2 all 16 patients with MIS-A had evidence of heart involvement and only 5 (31.3%) patients had skin rash. Like MIS-C, majority of MIS-A were reported in Hispanic or non-Hispanic Black.
The Centers for Disease Control and Prevention (CDC) published a case series of patients with MIS-A in October 2020.2 MIS-A was defined if included followings: 1) requiring hospitalization and age of ≥ 21; 2) laboratory evidence of current or previous SARS-CoV-2 infection; 3) severe dysfunction of one or more extrapulmonary organ systems; 4) severe elevated inflammatory markers; 5) absence of severe respiratory illness. Our case fulfilled all the CDC diagnosis criteria of MIS-A.
Because he reported a history of confirmed COVID-19, we could assess him as MIS-A. However, the history of confirmed COVID-19 could be absent. As the majority of COVID-19 in healthy patients is mild or asymptomatic,8 MIS-A patients might have not confirmed COVID-19 and that makes physicians difficult to consider the diagnose. In CDC reports,2 18.8% patients with MIS-A reported that they did not have previous respiratory illness and the initial SARS-COV-2 PCR test was negative in 37.5% of patients. Therefore, even having no history of COVID-19, it is necessary to suspect MIS-A if they had unknown etiology of shock along with fever, heart failure, and gastrointestinal symptoms. As MIS-A is the emergent condition, high index of suspicion is crucial and the SARS-CoV-2 antibody test could help the correct diagnosis.
In the previous report,3 20% and 6% of patients needed mechanical ventilation and extracorporeal membrane oxygenation, respectively, and mortalities have occurred in 4.1%. Our patient was admitted to the intensive care unit because of severe shock, receiving high dose of inotropes and vasopressors. Fortunately, he was discharged with a good response to the steroids and IVIG. The treatment guideline of MIS-A had not been established yet. Extrapolated from the treatment of Kawasaki disease and MIS-C, steroids and IVIG are suggested as the first-line treatment for MIS-A. In patients with MIS-C refractory to IVIG and steroid, anakinra, tocilizumab and infliximab have been tried.191011 Similar immunomodulating therapies are also tried in patients with MIS-A, although the data is more scarce. For example, tocilizumab which is an inhibitor of interleukin-6 was used in 13.7% of patients in the previous report.3
In conclusion, this is the first case of MIS-A in South Korea. Even there is no history of confirmed COVID-19, physicians should consider MIS-A as a differential diagnosis in patients with unknown etiology of shock, heart failure and gastrointestinal symptoms. Early diagnosis and prompt treatment of MIS-A are important to improve clinical outcomes. Steroid and IVIG remain as the effective first-line therapy.
Go to : 
References
1. Toubiana J, Poirault C, Corsia A, Bajolle F, Fourgeaud J, Angoulvant F, et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 2020; 369:m2094. PMID: 32493739.

2. Morris SB, Schwartz NG, Patel P, Abbo L, Beauchamps L, Balan S, et al. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 infection - United Kingdom and United States, March-August 2020. MMWR Morb Mortal Wkly Rep. 2020; 69(40):1450–1456. PMID: 33031361.

3. Bastug A, Aslaner H, Aybar Bilir Y, Kemirtlek N, Gursoy FM, Bastug S, et al. Multiple system inflammatory syndrome associated with SARS-CoV-2 infection in an adult and an adolescent. Rheumatol Int. 2021; 41(5):993–1008. PMID: 33742229.

4. Kim H, Shim JY, Ko JH, Yang A, Shim JW, Kim DS, et al. Multisystem inflammatory syndrome in children related to COVID-19: the first case in Korea. J Korean Med Sci. 2020; 35(43):e391. PMID: 33169560.

5. Lee JH, Han HS, Lee JK. The importance of early recognition, timely management, and the role of healthcare providers in multisystem inflammatory syndrome in children. J Korean Med Sci. 2021; 36(2):e17. PMID: 33429476.

6. Belay ED, Abrams J, Oster ME, Giovanni J, Pierce T, Meng L, et al. Trends in geographic and temporal distribution of US children with multisystem inflammatory syndrome during the COVID-19 pandemic. JAMA Pediatr. Forthcoming. 2021.

7. Davogustto GE, Clark DE, Hardison E, Yanis AH, Lowery BD, Halasa NB, et al. Characteristics associated with multisystem inflammatory syndrome among adults with SARS-CoV-2 infection. JAMA Netw Open. 2021; 4(5):e2110323. PMID: 34009351.

8. Yanes-Lane M, Winters N, Fregonese F, Bastos M, Perlman-Arrow S, Campbell JR, et al. Proportion of asymptomatic infection among COVID-19 positive persons and their transmission potential: a systematic review and meta-analysis. PLoS One. 2020; 15(11):e0241536. PMID: 33141862.

9. Lee PY, Day-Lewis M, Henderson LA, Friedman KG, Lo J, Roberts JE, et al. Distinct clinical and immunological features of SARS-CoV-2-induced multisystem inflammatory syndrome in children. J Clin Invest. 2020; 130(11):5942–5950. PMID: 32701511.

10. Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020; 324(3):259–269. PMID: 32511692.

11. Riollano-Cruz M, Akkoyun E, Briceno-Brito E, Kowalsky S, Reed J, Posada R, et al. Multisystem inflammatory syndrome in children related to COVID-19: a New York City experience. J Med Virol. 2021; 93(1):424–433. PMID: 32584487.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download