INTRODUCTION
Eosinophilic colitis (EC) is a rare clinical condition included in the group of eosinophilic gastrointestinal disorders (EGIDs) characterized by abnormal infiltration of the gastrointestinal mucosa by eosinophils in the absence of secondary causes.
1,2 EC is the least frequent manifestation of EGIDs with a bimodal age distribution. The condition appears to have a first peak in infants and a second peak in young adults. EC is a relatively common pathology in infants but rare in adults.
3,4 Furthermore, the epidemiologic features and pathophysiologic mechanisms have not been well defined.
Because the symptoms are similar to other gastrointestinal disorders, only a few case reports in which EC has been definitively diagnosed are available. Colitis dominated by eosinophilic infiltration may also occur secondarily in association with parasitic infections; inflammatory bowel disease; autoimmune diseases, such as systemic lupus erythematosus; drug reaction; and hypereosinophilic syndrome. All these secondary causes must be excluded before making a diagnosis of primary EC.
1,5,6 Although the standardized criteria have not been established, a diagnosis of EC is normally made by combining the clinical, endoscopy and histology findings. This paper presents a patient with uncommon endoscopic findings diagnosed with EC who was treated successfully with steroids.
Go to :

CASE REPORT
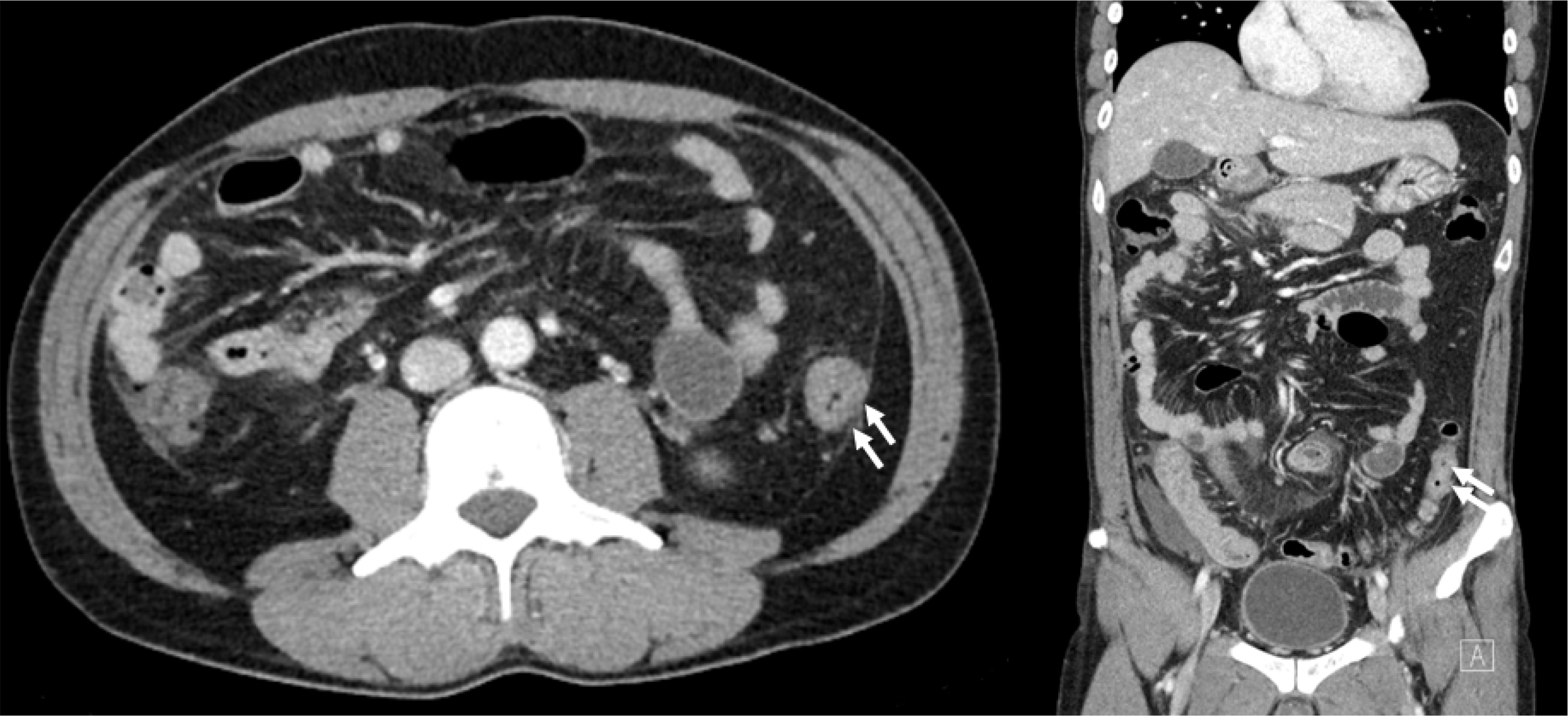
A 41-year-old man was admitted to hospital with complaints of diffuse abdominal pain and loose stools for 5 days. The frequency of loose stools increased to more than 5 times a day, and the characteristics of the stools were mushy, like fluffy pieces with ragged edges. The abdominal pain had become more aggravating in both lower quadrants the day before he was admitted. The patient had no medical history of severe disease and allergic reactions, such as rhinitis, asthma, sinusitis, dermatitis, food or drug allergies, or atopic conditions. The physical examination was unremarkable, except for lower abdominal mild tenderness. The patient showed no signs of end-organ damage beyond the gastrointestinal tract, such as the heart and skin. The laboratory findings were as follows: white blood cell count 15,100/μL (neutrophil 37%, lymphocyte 14%, monocyte 2%, eosinophil 47%), hemoglobin 14.9 g/dL, and platelet count 254,000/μL. The eosinophil count was elevated to 7,097/μL (reference 0-800/μL). The erythrocyte sedimentation rate (ESR) was elevated at 30 mm/hours, and the CRP was modestly elevated at 1.40 mg/dL. The antibodies against Clonorchis sinensis and Paragonimus westermani were negative. The anti-nuclear cytoplasmic antibody and anti- Saccharomyces cerevisiae antibody were negative. The serum IgE levels were normal, and the fecal parasite test results were negative. A CT scan of the abdomen showed only nonspecific colitis with mild wall thickening of the descending colon and small fluid collections in the lower abdominopelvic cavity (
Fig. 1). The CT scan did not reveal any other subepithelial tumor-like lesions except for mild enterocolitis. Nevertheless, the colonoscopy showed multiple whitish subepithelial tumor-like lesions from the cecum to the rectum with erythema and mucosal edema (
Fig. 2). Multiple biopsies were taken from each segment of the colon and rectum. The histopathology examination revealed eosinophilic inflammatory infiltrates in the lamina propria from all segments of the biopsies (
Fig. 3). There were >160 eosinophils per high power field. The examination corresponded to mucosal involvement of the intestinal layers, and his clinical features were the most common forms of EC without severe complications. Prednisolone 30 mg/day was started; the calculated dose was 0.5 mg/kg/day because his body weight was 60 kg. His symptoms resolved within a week. Prednisolone was tapered by 5 mg weekly until a 5 mg/day dose was reached in the last week. A follow-up colonoscopy was performed 2 weeks after he was given the last dose, and the findings were normal (
Fig. 4). The patient was followed up as an outpatient for 6 months with no recurrence of his symptoms.
 | Fig. 1Computed tomography findings were nonspecific. Mild wall thickening with mucosal enhancement in the descending colon. 
|
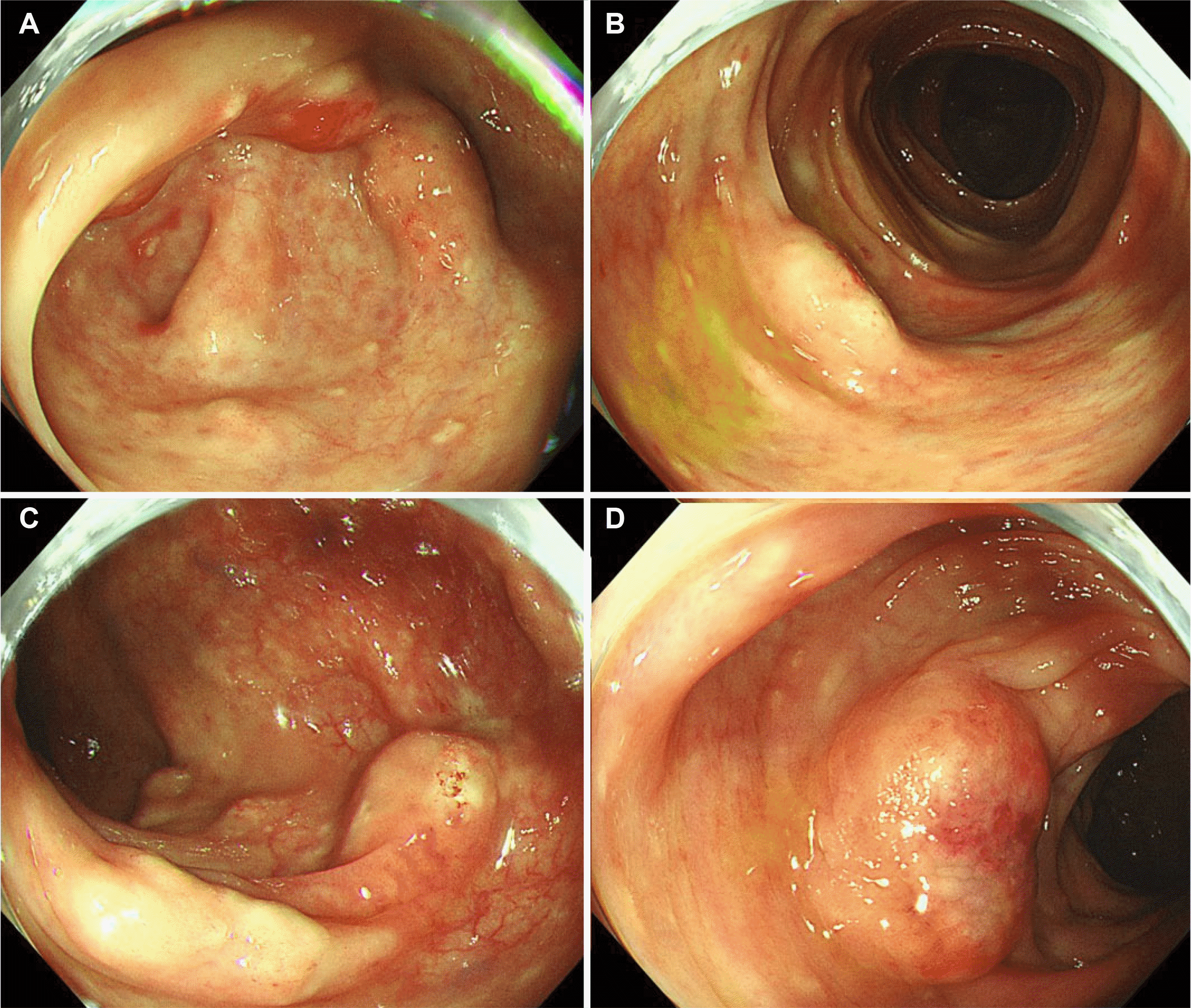 | Fig. 2Colonoscopy findings. Multiple whitish subepithelial tumor-like lesions with nonspecific inflammatory changes, such as erythema and mucosal edema, were observed. (A) Cecum. (B) Transverse colon. (C) Descending colon. (D) Sigmoid colon. 
|
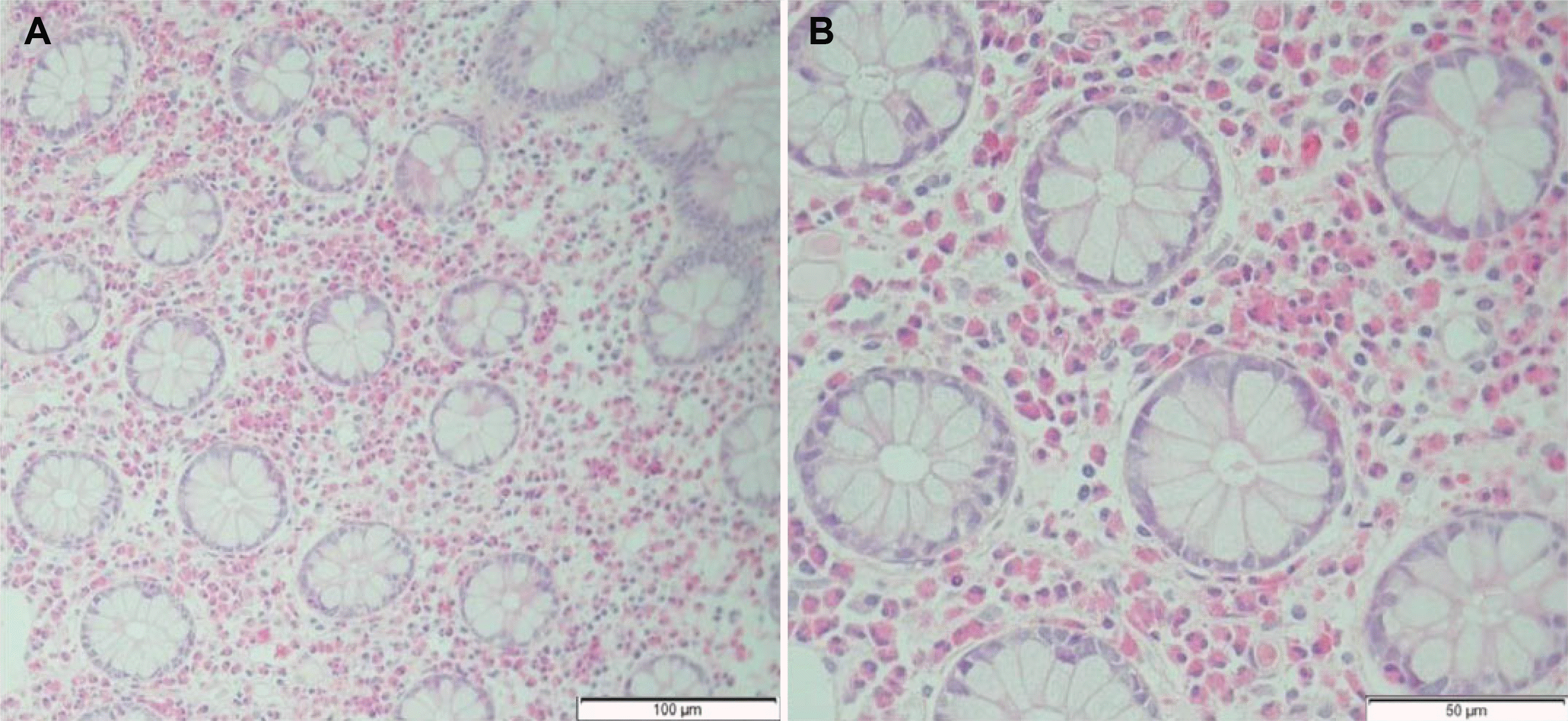 | Fig. 3Pathology findings. Numerous eosinophils in the lamina propria of the colonic mucosa. (A) H&E staining, ×200. (B) H&E staining, ×400. 
|
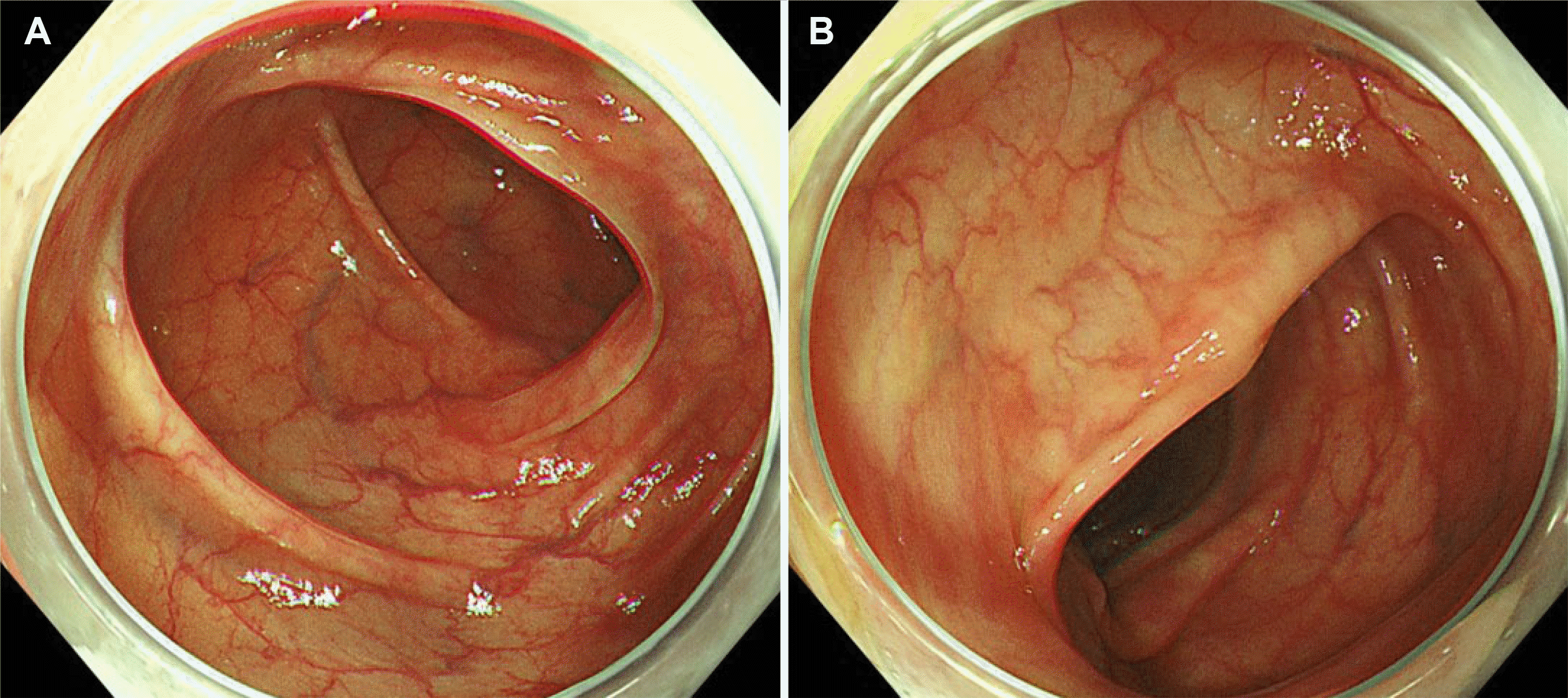 | Fig. 4Follow up endoscopy findings. The subepithelial tumor-like lesions were improved compared to the previous colonoscopy. (A) Descending colon. (B) Sigmoid colon. 
|
Go to :

DISCUSSION
EC is a rare inflammatory disease that infrequently affects the colonic mucosa macroscopically and can be underdiagnosed.
7 The histology findings will lead to a definitive diagnosis if other causes can be excluded. Secondary conditions that lead to the eosinophilic infiltration of the colonic mucosa need to be excluded to establish a correct diagnosis.
1,5,6
Although the etiology and pathophysiology of this disease are not entirely understood, an interaction between genetic and environmental factors has been implicated. EC is associated with a wide spectrum of allergic diseases, such as rhinitis, asthma, sinusitis, dermatitis, eczema, and urticaria.
3,8 In most cases, primary EC is related to an allergic reaction, either an IgE-mediated anaphylactic-type food allergy or a food enteropathy not mediated by IgE. In infants, EC appears to be an IgE-associated disorder with mast cell accumulation and degranulation in the colonic tissue. On the other hand, in adults, EC is more commonly a non-IgE-mediated allergic reaction associated with a delayed CD4(+) Th2 lymphocytes response.
1,9
The clinical features depend on the intestinal layers affected by the eosinophilic infiltration. Mucosal involvement is the most common form and presents with diarrhea, malabsorption, and protein-losing enteropathy.
10 Transmural disease has a more severe presentation with wall thickening or strictures that can lead to intestinal obstruction, volvulus, and perforation.
11 Subserosal disease, a very rare form, presents with eosinophilic ascites and is associated with a good prognosis.
12 Endoscopic biopsies may be non-diagnostic in cases of muscular or subserosal involvement. Because endoscopic biopsies do not sample the submucosa, muscularis propria, or serosa, it is difficult to distinguish between the various forms.
4
The laboratory findings might be indicative of EC, but they are typically not sufficient for a diagnosis. The blood eosinophil count can be normal in up to 20% of patients. The radiological findings are often nonspecific and only present in 60-70% of cases in adults. CT imaging may show nodularity of the bowel wall, colonic wall thickening, mucosal fold thickening, and ascites in some cases.
13,14 Imaging studies might help exclude inflammatory and infectious colitis.
The endoscopic findings are variable and usually nonspecific, such as edematous mucosa with a loss of the normal vascular pattern, patchy erythematous changes, erosions, or aphthous ulcerations.
15,16 Although endoscopic studies are frequently associated with normal macroscopic findings, microscopically, EC shows diffuse patchy involvement. Therefore, multiple endoscopic biopsies are required if EC is suspected despite the visualization of a normal mucosa. EC is diagnosed by the appearance of sheets or clusters of eosinophils in the lamina propria. On the other hand, there are no guidelines that define an excessive versus normal number of eosinophils in the colonic mucosa.
7
EC tends to be more aggressive in adolescents and adults, whereas it is relatively benign in infants and usually resolves within days after removing the triggering food. Adolescents and adults often require more aggressive medical management.
1 Several treatment options are available, even though the evidence for most is limited to a few case studies.
Corticosteroids are used as first-line pharmacological therapy if a dietary therapy fails to achieve an adequate clinical response. Oral prednisone at doses of 20-40 mg per day for 2 weeks has been shown to induce clinical remission in most patients.
17 On the other hand, the main problem with corticosteroids is the high relapse rate. Maintenance treatment might be required in patients whose symptoms relapse during or after drug tapering with low-dose prednisone or budesonide.
10
In severe or recurrent cases, long courses of steroids or immunomodulatory agents are needed. Mesalazine has been reported to be effective in some cases.
2 Azathioprine and anti- tumor necrosis factor agents, such as infliximab and adalimumab, have been attempted in the severe, steroid-refractory, or steroid-dependent EC.
18,19 Other steroid-sparing agents can be an option, including antihistamines, mast-cell stabilizers, and leukotriene receptor antagonists, even though their effectiveness in EC has yet to be evaluated.
1,20
In summary, this paper presented a case of EC with the endoscopic appearance of subepithelial tumor-like lesions, which were proven to be eosinophilic infiltrates by multiple biopsies. In the absence of clear diagnostic guidelines, EC is diagnosed primarily by exclusion. Therefore, the most important factor in making a correct diagnosis is having a high degree of clinical suspicion.
Go to :







 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download