This article has been
cited by other articles in ScienceCentral.
Abstract
Catastrophic antiphospholipid syndrome is a highly fatal condition characterized by widespread thromboembolism subsequent to a triggering factor (e.g., infection, trauma, and neoplasia) in antiphospholipid antibody-positive patients. This paper reports a case of a 29-year-old male without the underlying disease who developed extensive mesenteric thromboembolism and jejunal necrosis during the treatment for acute enteritis. The patient’s condition was improved with low-molecular-weight heparin and an intravenous Ig treatment with emergency surgery. The serum antiphospholipid (anticardiolipin IgM) and lupus anticoagulant antibody tests showed positive results. Acute infectious enterocolitis is generally considered a mild disease. On the other hand, aggressive evaluation and treatment should be considered if the clinical conditions do not improve and deteriorate rapidly despite appropriate antibiotic treatment because of the possibility of acute immunological complications, such as catastrophic antiphospholipid syndrome.
Go to :

Keywords: Catastrophic antiphospholipid syndrome, Mesenteric ischemia, Intestine, small, Surgical procedure, Enterocolitis
INTRODUCTION
Catastrophic antiphospholipid antibody syndrome (CAPS) is an autoimmune disease associated with multi-organ failure, accounting for less than 1% of all cases of antiphospholipid antibody syndrome (APS). Antiphospholipid antibodies are present in 14% of stroke cases, 11% of myocardial infarction cases, 10% of deep vein thrombosis cases, and 9% of infertility cases.
1 On the other hand, abdominal symptoms in APS are rare, with only 1.5% of APS cases displaying symptoms.
2 Microthrombi are found through an autopsy in most CAPS patients, and the presence of these microthrombi is one of the factors distinguishing APS from CAPS. APS is characterized by a single vein or arterial occlusion of the medium and large blood vessels. On the other hand, CAPS is predominant in diffuse small-vessel ischemia and thrombosis affecting the function of the major organs.
3
The mortality rate in CAPS ranges from 37% to 50%. CAPS progresses rapidly and is difficult to distinguish from other diseases. Therefore, early diagnosis and treatment are essential for survival.
4,5 Gastrointestinal ischemia has been reported in 14-38% of CAPS patients.
6-9 In a study of 80 patients diagnosed with CAPS, 18 patients (22%) had abdominal pain as the initial major symptom.
10 The triggers of CAPS generally include infection, surgery, malignancies, and discontinuation of therapeutic anticoagulant treatment, which could be identified in only 65% of CAPS cases.
4,5,11 This paper presents a case of severe sepsis with simple colitis, causing CAPS, resulting in severe small intestinal necrosis.
Go to :

CASE REPORT
A 29-year-old man, who had abdominal pain and diarrhea that started 10 days earlier, was diagnosed with acute colitis in the ascending colon by CT, possibly caused by infection at another hospital. During hospitalization, his symptoms were not resolved after intravenous antibiotic therapy with massive hydration for acute colitis. A blood test was performed, and the antibiotics were escalated to treat the severe colitis that was refractory to conventional antibiotics. He denied a history of other gastrointestinal diseases, as well as the possibility of eating spoiled foods. No definite evidence of bacterial or viral infections was present based on stool analysis and blood culture. His symptoms did not improve after increasing antibiotic therapy. Therefore, he refused further treatment and was discharged on the 6th day of hospitalization. Two days after discharge, he was admitted to the emergency room of the Ewha Womans University Mokdong Hospital because of persistent abdominal pain. At the time of admission, his stool frequency was 2 or 3 times the normal frequency, and right upper quadrant pain was measured as mild on the pain scale at the initial evaluation. His symptoms were slightly better than the initial ones. The clinical severity was not high. He had never smoked and did not drink alcohol. He had no underlying disease and denied any family history of inflammatory bowel disease, tuberculosis, and autoimmune disease. The laboratory findings at the time of admission to Ewha Womans University Mokdong Hospital were as follows: white blood cell count 12.740/μL; hemoglobin 14.6 g/dL; platelet count 65,000/μL; CRP 4.1 mg/dL; fibrinogen 281.5 mg/L; fibrinogen degradation production 68.4 mg/L; D-dimer, 29.48 mg/L. His liver function tests and the estimated glomerular filtration rate were normal. The results of stool multiplex PCR and cultures were negative. Culture and PCR for the detection of
clostridium difficile toxin were also negative. The initial sigmoidoscopic examination revealed generalized mucosal edema with focal hyperemic changes from the rectum to the distal sigmoid colon (
Fig. 1). Because clinical information from other hospitals was unavailable, empirical intravenous ciprofloxacin with metronidazole therapy was restarted at the time of admission.
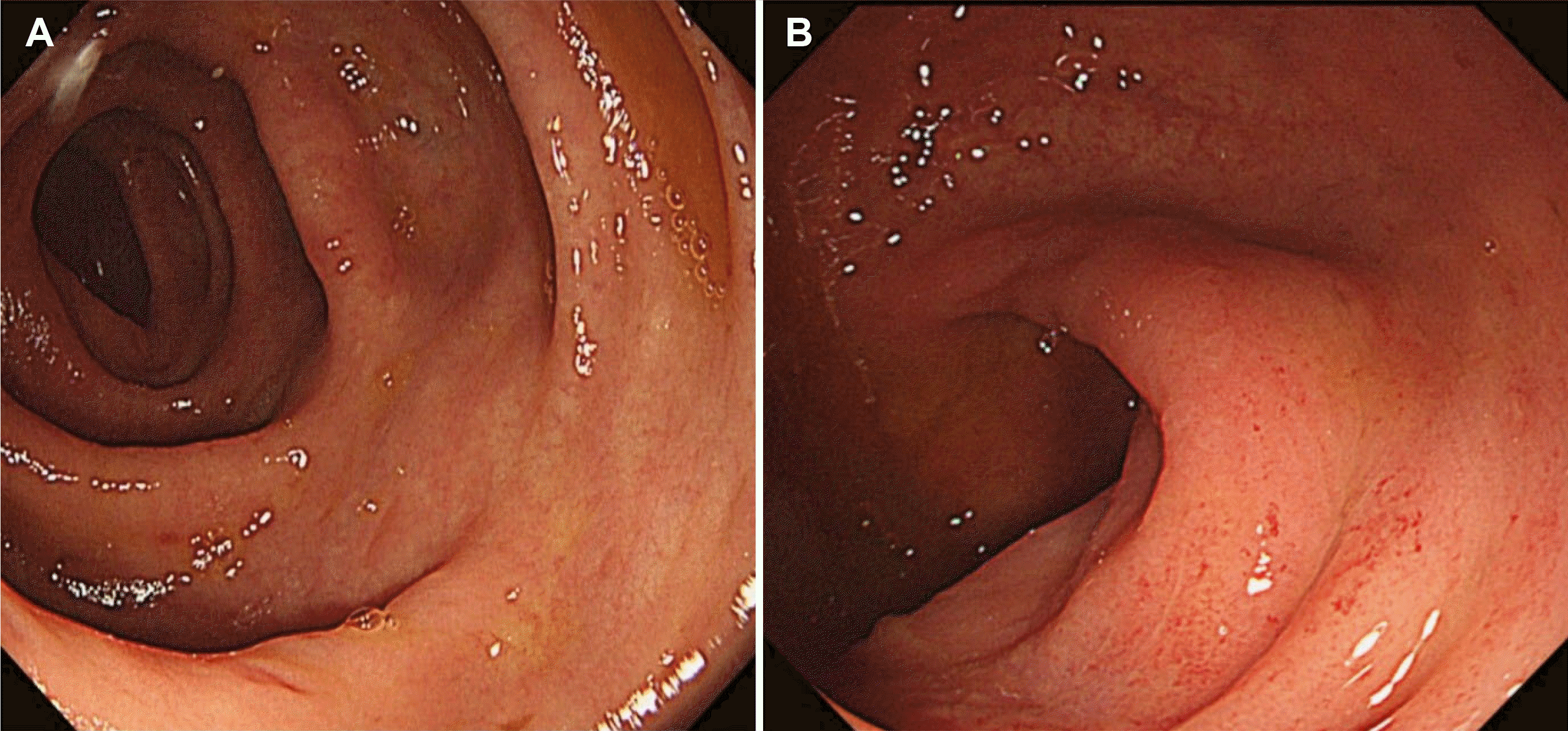 | Fig. 1Sigmoidoscopic examination. (A) Mucosal edema with (B) focal hyperemic changes in the sigmoid and rectal colon. 
|
After 3 days of antibiotic therapy, he showed a sudden worsening of the abdominal symptoms, including high fever and severe abdominal pain without diarrhea. Finally, he presented with hematochezia, hypotension, and a drowsy mentality. His vital signs at that time were as follows: body temperature, 38.0℃; blood pressure, 75/36 mmHg; heart rate, 120/min; respiratory rate, 18/min. The physical examination revealed severe direct tenderness around the right upper quadrant abdomen. The laboratory results were as follows: white blood cell count 17,680/μL; hemoglobin 14.2 g/dL; platelet count, 47,000/μL; CRP 10.70 mg/dL (<0.5 mg/dL); fibrinogen 223.4 mg/dL (180-350 mg/dL); fibrinogen degradation production >80 μg/mg (<5 μg/mg); D-dimer, 35.2 mg/L fibrinogen-equivalent units (<0.59 mg/L fibrinogen-equivalent units). Follow-up CT of the abdomen and pelvis revealed multiple thrombi in the main portal vein, right portal vein, left portal vein, splenic vein, superior mesenteric vein, abdominal aorta, and right common iliac artery, and a spleen infarction and ischemia in most of the jejunum. Unlike the simple enteritis detected on CT in the emergency room 3 days earlier, these were acute and worse (
Fig. 2). With a large amount of hydration and repeated blood transfusion, low-molecular-weight heparin (LMWH) and intravenous Ig (IVIG) therapies were started to prevent the spread of thrombi with the development of CAPS. The patient showed hematochezia during the medical treatment. An emergency exploratory laparotomy with a jejunal excision and duodeno-ileostomy was performed due to extensive necrosis from the proximal jejunum to the distal jejunum (
Fig. 3). The histology examination of the biopsy specimens revealed ischemic necrosis in the mucosal layer, acute serositis, and submucosal and subserosal congestion with thrombi in the small vessels of the small bowel and mesentery (
Fig. 4). After surgery, the blood test revealed a positive result for serum lupus anticoagulant antibodies and a weakly positive anticardiolipin antibody IgM level of 13.6 MPL U/mL (normal range: <10 MPL U/mL).
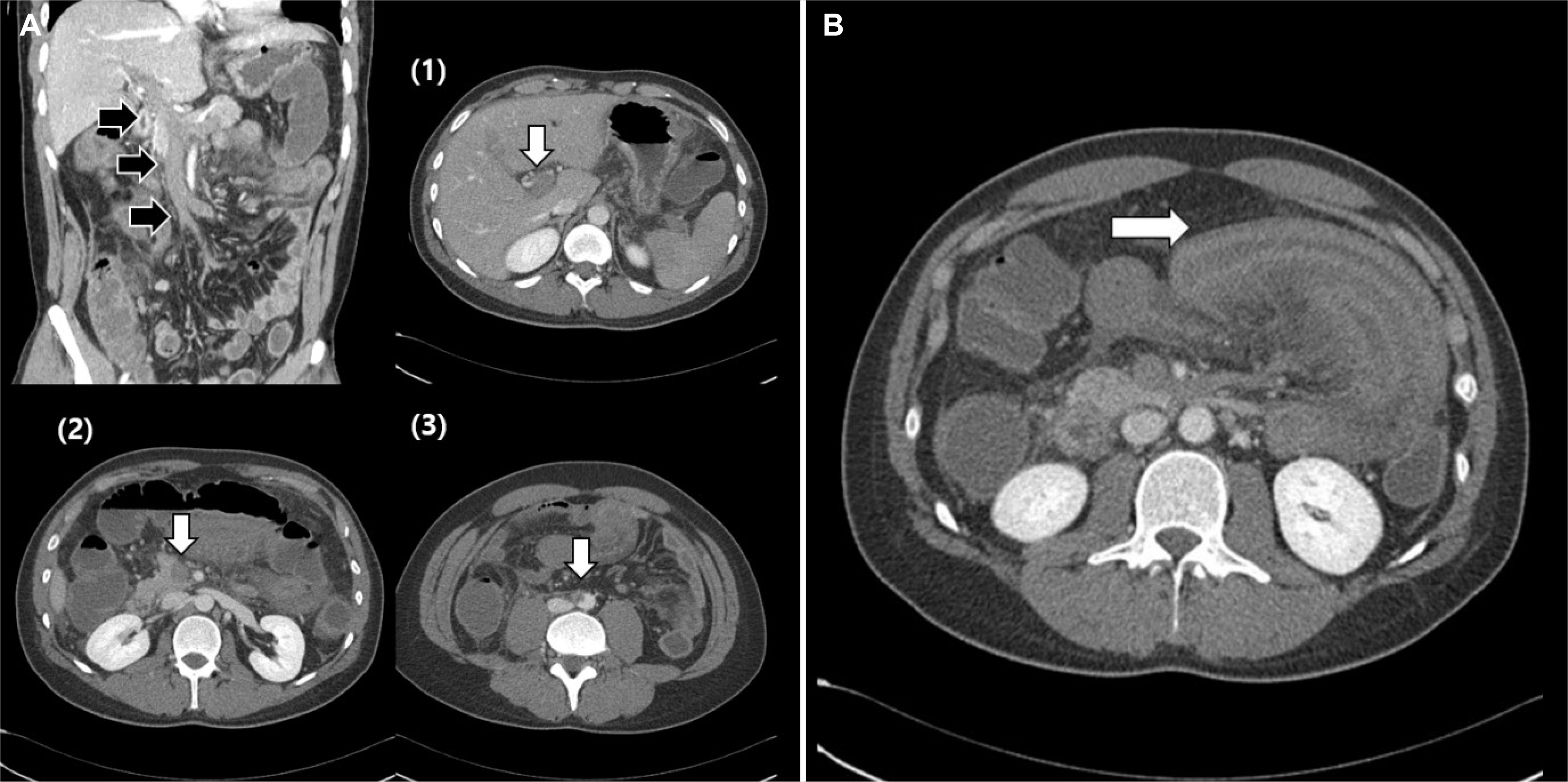 | Fig. 2Contrast-enhanced computed tomography of the abdomen. (A) Multiple thrombi (arrows; (1) main portal vein, (2) superior mesenteric artery, (3) abdominal aorta) and (B) segmental dilated small bowel loops with wall thickening and ischemia (arrow). 
|
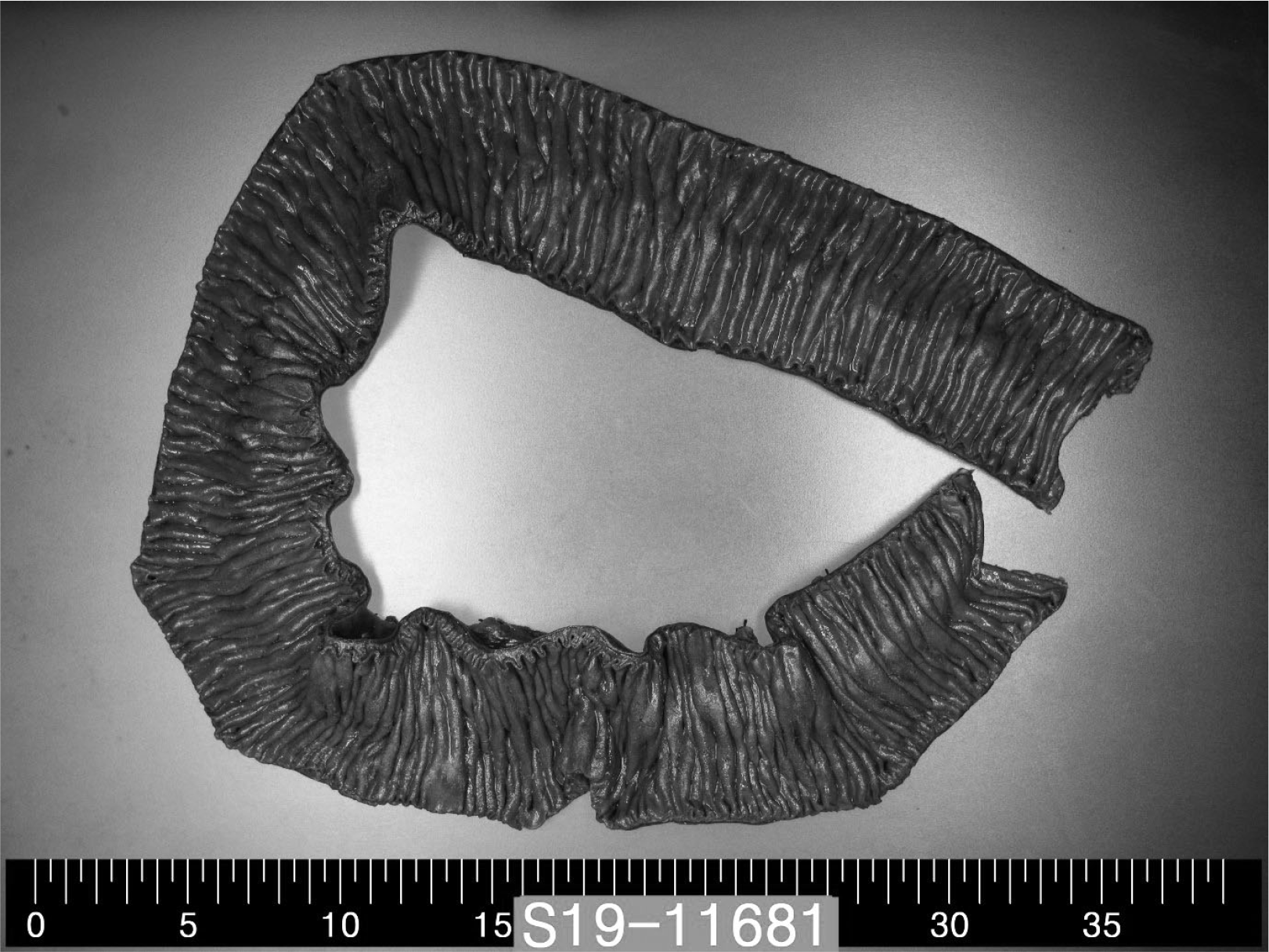 | Fig. 3Gross appearance of a resected necrotic jejunum with no disappearance of mucosal folds or color change. A subtle ischemic change was predicted. 
|
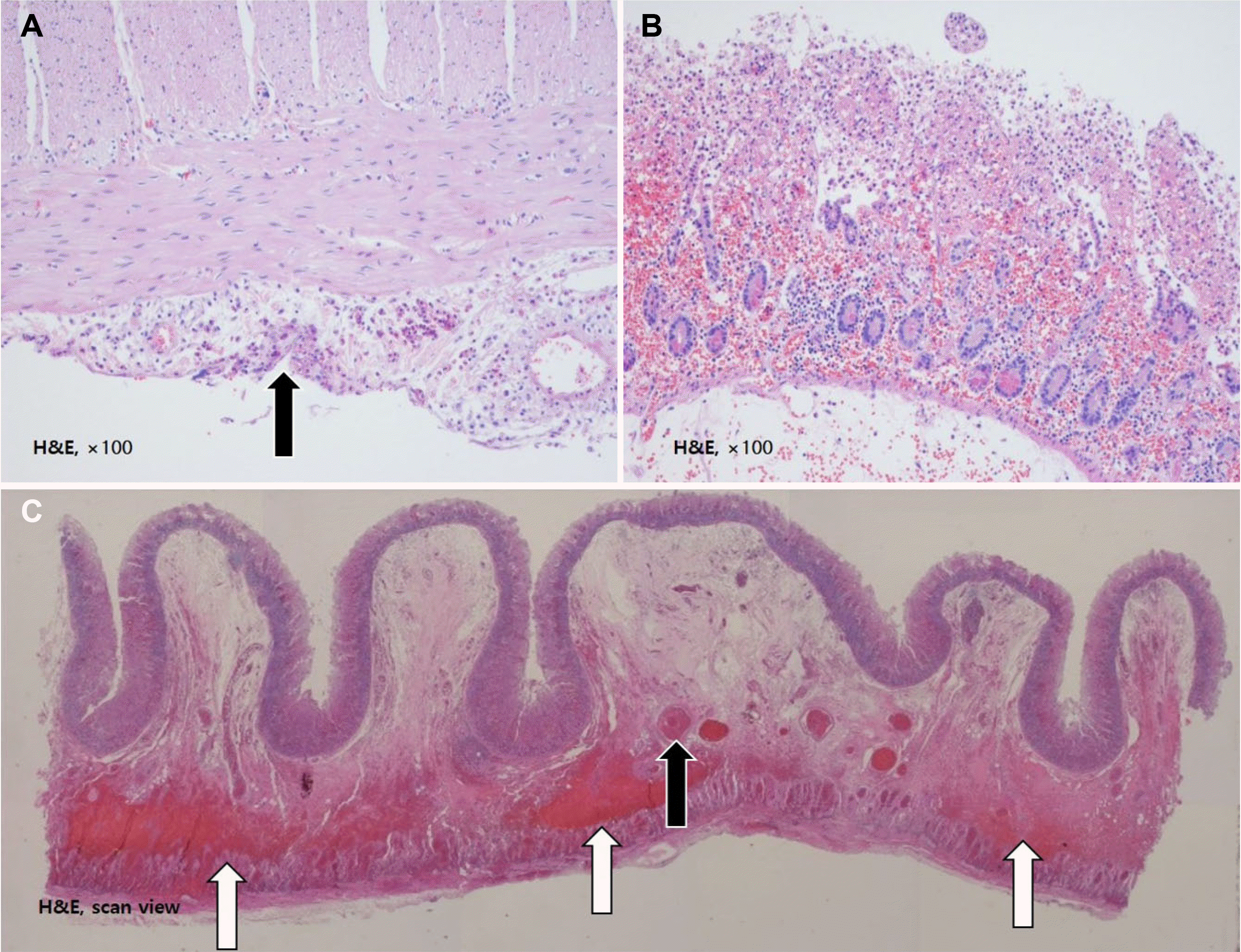 | Fig. 4Pathology findings. Hematoxylin-eosin (H&E) staining revealed (A) the serosal infiltration of inflammatory cells (arrow); serositis (H&E, ×100). (B) Ischemic necrosis of the mucosae (H&E, ×100). (C) Thrombosis of small vessels in the small bowel mesentery (black arrow) and congestion (white arrows) (H&E, ×40). 
|
After the emergency operation, his clinical condition was improved gradually while maintaining intravenous antibiotics, IVIG, and LMWH with repeated platelet transfusion. A small amount of hematochezia was noted on the 7th day after the operation, but it resolved quickly after conservative treatment without blood transfusion. Finally, he was discharged after 15 days of hospitalization.
Approximately 4 weeks after the initiation of anticoagulation therapy, the abdominal CT findings were as follows: interval improvement of extensive thrombosis in the main portal vein, right portal vein, left portal vein, splenic vein, superior mesenteric vein, and azygous vein with a residual lesion; interval improvement of thrombosis in the abdominal aorta (just below the inferior mesenteric artery orifice) and right common iliac artery with a residual lesion. Based on the CT results, continuous anticoagulation therapy was required. Nevertheless, the patient was lost to follow-up, and no further testing and treatment could be performed after 4 weeks from discharge.
Go to :

DISCUSSION
This paper reports a case of severe sepsis triggered by small bowel necrosis with colitis indicated by abdominal pain and diarrhea in CAPS. The criteria for the definitive diagnosis of CAPS include the following: 1) involvement of three or more organ systems or tissues; 2) development of manifestations simultaneously or in less than a week; 3) histopathology confirmation of a small vessel occlusion in at least one organ or tissue; and 4) laboratory confirmation of the presence of antiphospholipid antibodies (i.e., lupus anticoagulant or anticardiolipin antibodies). In this case, the disease progressed within 3 days. Anticardiolipin IgM was positive. Three organs, including the large intestine, small intestine, and spleen, were invaded, and a histology examination revealed infarction in the small intestine. Positive results for antiphospholipid antibodies at 6 weeks after the initial episode are required for the definite diagnosis of CAPS. Although it was not possible to confirm CAPS due to follow-up loss, CAPS was highly likely. The guidelines suggested that flexibility is important considering that a patient could become seriously ill with the involvement of just one or two major organs. In addition, the histological examination requires a substantial amount of time and equipment, and the patient might die before the results of antiphospholipid antibody tests provide confirmation. Some researchers proposed that clinicians should immediately start aggressive treatment according to the guidelines for CAPS treatment in some cases if they believe that the symptoms are “CAPS-like” despite not meeting the precise definition.
12
In a review of patients with CAPS, abdominal manifestations of CAPD were mainly cases with hepatic involvement (34%), including Budd-Chiari syndrome, hepatic-veno-occlusive disease, occlusion of small hepatic veins, or hepatic infarction; 18% of cases involved an intestinal infarction. Furthermore, symptoms involving the spleen and pancreas were rarely reported.
3 If there is intestinal involvement, the clinical manifestation is usually presented as abdominal pain or gastrointestinal bleeding due to bowel ischemia.
13 Upper gastrointestinal bleeding is also possible if the extent of the infarct is wide.
14 The mortality for acute mesenteric ischemia is approximately 40%.
15 The prognostic factors that can predict mortality in patients with CAPS are cerebral (mainly stroke) involvement, cardiac involvement, and infection; these factors are the leading causes of death. The presence of SLE may also increase the mortality risk.
3
No randomized trials have been performed to determine the optimal treatment for CAPS because the disease is very rare. On the other hand, a large case study provided an outline of the possible treatment. Importantly, any precipitating factor should be treated, and necrotic tissues should be resected.
6,10,16,17 Eculizumab and rituximab may be considered for refractory cases. Positive outcomes with the first use of eculizumab have been observed in several recent studies.
18-20 Nevertheless, the standard medical therapy is plasma separation, or IVIG added to a combination of anticoagulants and glucocorticoids. The maximum recovery rate was increased by approximately 70-30% when anticoagulants and corticosteroids were combined with plasmapheresis.
3 Therefore, suspected patients should consult with specialists to expedite treatment, and early admission to the intensive care unit is crucial.
In the initial therapy for the patient, the anticoagulant LMWH (1 mg/Wt/day) was delivered subcutaneously. Corticosteroids may be given along with heparin because steroids can minimize complications from tissue loss (necrosis) often accompanied by CAPS. The patient had septic and bleeding episodes; thus, steroids were not used for the initial management due to his worsening clinical condition. IVIG (0.4 g/kg/day) was also used based on consultation with a rheumatologist. Intensive antibiotic therapy was initiated to control the severe infection. The patient did not undergo time-consuming plasmapheresis because the main cause of CAPS was sepsis from a gastrointestinal infection, and emergency surgery was the primary solution for this infection. Anticoagulant therapy and surgery are difficult for initial treatment when a patient with thrombocytopenia also has hematochezia. On the other hand, considering the mechanism of the disease, this treatment is critical; thus, anticoagulant therapy was rapidly started. Repeated bleeding was controlled with a large amount of blood transfusion with close monitoring.
In this case, the disease started with symptoms of acute enterocolitis, including abdominal pain and diarrhea. The clinical course became worse despite fasting and antibiotic treatment. The occurrence of severe diseases was difficult to predict initially because the patient was a young and healthy person with no risk factors for arteriosclerosis or other diseases before the onset of symptoms. This case demonstrated that although acute infectious enterocolitis is generally considered a mild disease, aggressive evaluation and treatment should be considered if the clinical condition does not improve and deteriorates rapidly despite appropriate antibiotic treatment because of the possibility of acute immunological complications, such as CAPS.
Go to :








 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download