1. Ahn JH. An update on the role of bronchoscopy in the diagnosis of pulmonary disease. Yeungnam Univ J Med. 2020; 37(4):253–261. PMID:
32891075.

2. Anantham D, Koh MS, Ernst A. Endobronchial ultrasound. Respir Med. 2009; 103(10):1406–1414. PMID:
19447014.

3. Kurimoto N, Miyazawa T, Okimasa S, Maeda A, Oiwa H, Miyazu Y, et al. Endobronchial ultrasonography using a guide sheath increases the ability to diagnose peripheral pulmonary lesions endoscopically. Chest. 2004; 126(3):959–965. PMID:
15364779.

4. Steinfort DP, Khor YH, Manser RL, Irving LB. Radial probe endobronchial ultrasound for the diagnosis of peripheral lung cancer: systematic review and meta-analysis. Eur Respir J. 2011; 37(4):902–910. PMID:
20693253.

5. Ali MS, Trick W, Mba BI, Mohananey D, Sethi J, Musani AI. Radial endobronchial ultrasound for the diagnosis of peripheral pulmonary lesions: a systematic review and meta-analysis. Respirology. 2017; 22(3):443–453. PMID:
28177181.

6. Chen CH, Cheng WC, Wu BR, Chen CY, Chen WC, Hsia TC, et al. Improved diagnostic yield of bronchoscopy in peripheral pulmonary lesions: combination of radial probe endobronchial ultrasound and rapid on-site evaluation. J Thorac Dis. 2015; 7(Suppl 4):S418–S425. PMID:
26807290.
7. Moon SM, Choe J, Jeong BH, Um SW, Kim H, Kwon OJ, et al. Diagnostic performance of radial probe endobronchial ultrasound without a guide-sheath and the feasibility of molecular analysis. Tuberc Respir Dis (Seoul). 2019; 82(4):319–327. PMID:
31172704.

8. Asano F, Eberhardt R, Herth FJ. Virtual bronchoscopic navigation for peripheral pulmonary lesions. Respiration. 2014; 88(5):430–440. PMID:
25402610.

9. Minezawa T, Okamura T, Yatsuya H, Yamamoto N, Morikawa S, Yamaguchi T, et al. Bronchus sign on thin-section computed tomography is a powerful predictive factor for successful transbronchial biopsy using endobronchial ultrasound with a guide sheath for small peripheral lung lesions: a retrospective observational study. BMC Med Imaging. 2015; 15(1):21. PMID:
26092497.

10. Tay JH, Irving L, Antippa P, Steinfort DP. Radial probe endobronchial ultrasound: factors influencing visualization yield of peripheral pulmonary lesions. Respirology. 2013; 18(1):185–190. PMID:
23035636.

11. Evison M, Crosbie PA, Morris J, Martin J, Barber PV, Booton R. Can computed tomography characteristics predict outcomes in patients undergoing radial endobronchial ultrasound-guided biopsy of peripheral lung lesions? J Thorac Oncol. 2014; 9(9):1393–1397. PMID:
25122434.

12. Park S, Yoon HY, Han Y, Wang KS, Park SY, Ryu YJ, et al. Diagnostic yield of additional conventional transbronchial lung biopsy following radial endobronchial ultrasound lung biopsy for peripheral pulmonary lesions. Thorac Cancer. 2020; 11(6):1639–1646. PMID:
32342673.

13. Ali MS, Sethi J, Taneja A, Musani A, Maldonado F. Computed tomography bronchus sign and the diagnostic yield of guided bronchoscopy for peripheral pulmonary lesions. A systematic review and meta-analysis. Ann Am Thorac Soc. 2018; 15(8):978–987. PMID:
29877715.

14. Chen AC, Loiselle A, Zhou L, Baty J, Misselhorn D. Localization of peripheral pulmonary lesions using a method of computed tomography-anatomic correlation and radial probe endobronchial ultrasound confirmation. Ann Am Thorac Soc. 2016; 13(9):1586–1592. PMID:
27388116.

15. Lee KM, Lee G, Kim A, Mok J, Lee JW, Jeong YJ, et al. Clinical outcomes of radial probe endobronchial ultrasound using a guide sheath for diagnosis of peripheral lung lesions in patients with pulmonary emphysema. Respir Res. 2019; 20(1):177. PMID:
31387600.

16. Good WR, Christensen PM, Herath S, Dawkins P, Yap E. Radial-probe endobronchial ultrasound outcomes in the investigation of peripheral pulmonary lesions: a New Zealand perspective. Intern Med J. 2018; 48(12):1481–1487. PMID:
30091278.

17. Zhang Q, Zhang S, Xu X, Xu Q, Zhou J. Value of radial probe endobronchial ultrasound-guided transbronchial biopsy and computer tomography-guided transthoracic needle aspiration in the diagnosis of peripheral pulmonary lesions. Medicine (Baltimore). 2017; 96(34):e7843. PMID:
28834894.

18. Huang CT, Ho CC, Tsai YJ, Yu CJ, Yang PC. Factors influencing visibility and diagnostic yield of transbronchial biopsy using endobronchial ultrasound in peripheral pulmonary lesions. Respirology. 2009; 14(6):859–864. PMID:
19703067.

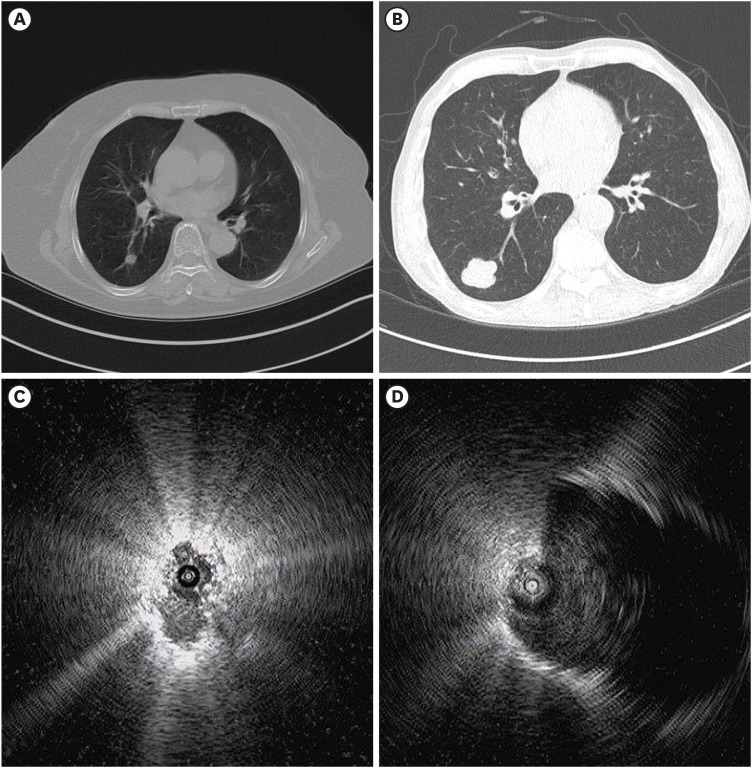
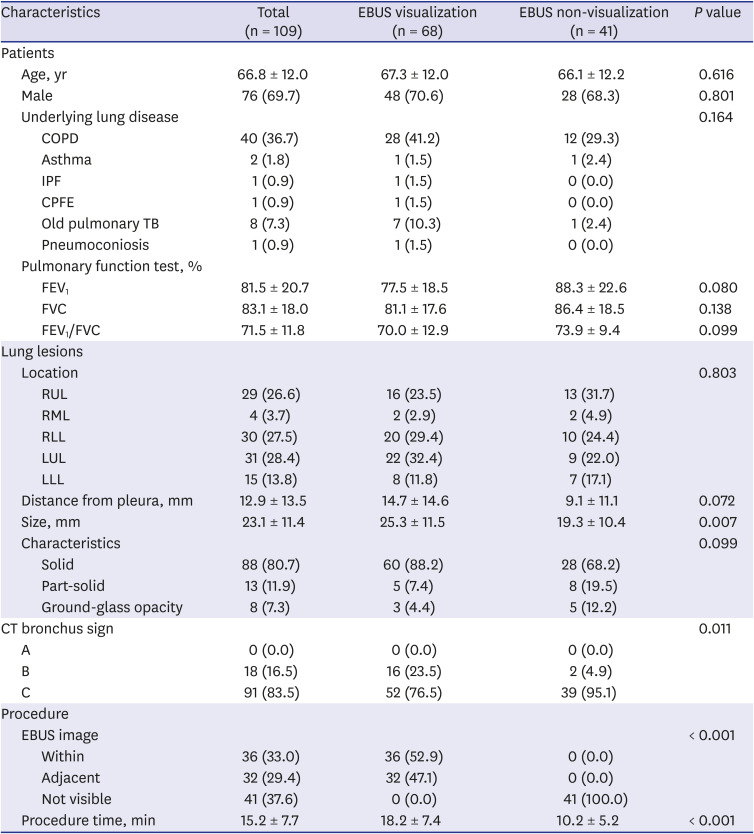
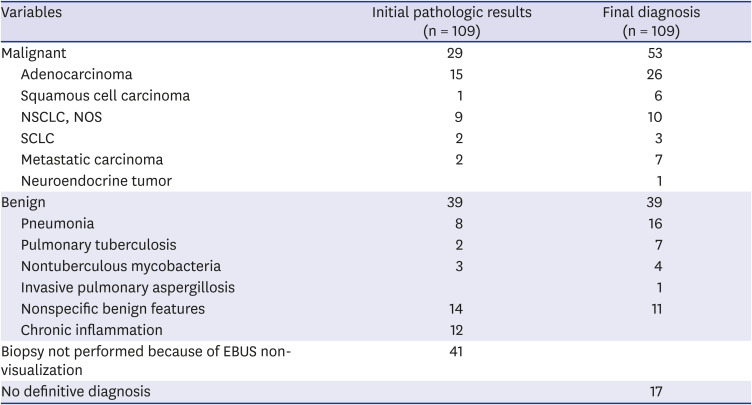
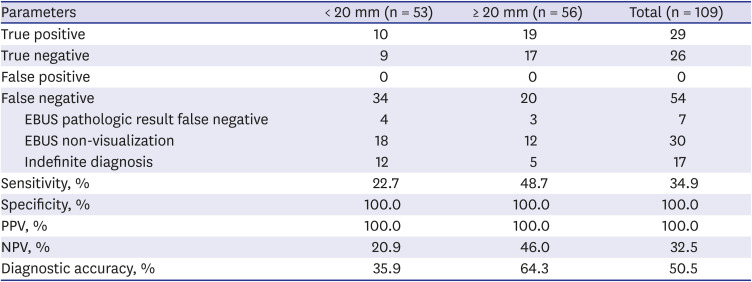




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download