Abstract
Objectives
It is well known that type 2 diabetes (T2DM) is dramatically improved after bariatric surgery, although the mechanisms have not been clearly identified. The skill required for gastric surgery for gastric cancer is very similar to that needed in bariatric surgery. In this study, we evaluated the immediate improvement of T2DM after gastrectomy for gastric cancer.
Methods
A total of nine patients who were diagnosed with early gastric cancer (EGC) and already had T2DM underwent a 75 g oral glucose tolerance test (OGTT) before surgery and within two weeks after gastrectomy. Glucose, insulin, and c-peptide were measured before, and 30 and 60 minutes after ingesting 75 g of glucose. From these trials, we calculated the HOMA-IR, insulinogenic index, Matsuda index, and area under the curve (AUC).
Results
The mean age of participants was 57.23 ± 11.08 years and eight of them were men. HOMA-IR (4.2 vs. 2.3, P = 0.012) levels were decreased after surgery. There were no significant differences of insulinogenic index, fasting blood sugar before and after surgery. The Matsuda index (3.3 vs. 8.3, P = 0.002) was significantly increased and AUC (512.9 vs. 388.7 mg-hr/dL, P > 0.001) upon 75 g OGTT was significantly decreased after surgery.
Go to : 
The surgical procedures for stomach cancer and obesity are very similar. Both include resection of stomach and bypass of part of the proximal intestine. Previous studies showed improvement of diabetes after gastrectomy for stomach cancer patients. One retrospective study reported T2DM was cured in 15.1% and was improved in 30.4% of individuals after stomach cancer surgery.1 Another study showed an improving effect for diabetes; 85% of patients that underwent Billroth-II (B-II) surgery showed improvement, and 88% of those receiving Roux-En-Y gastric bypass (RYGB) showed improvement.2
However, these results have an important bias. Almost all patients change their lifestyle after being diagnosed with cancer. They stop smoking and drinking alcohol, eat healthier food, and exercise more. Further, almost all patients report weight loss after gastric cancer surgery, unlike other procedures. These differences can lead to the improvement of diabetes.
In cases of metabolic surgery, improvement of diabetes occurs by both weight-dependent and - independent mechanisms. The most powerful evidence of weight-independent mechanism is the improvement of hyperglycemia that occurs rapidly after surgery before patients observe weight loss.3 Moreover, glycemic improvement was noted even in the mild obesity group, and not only in the severe obesity group.4
Therefore, we hypothesized that immediate improvement of insulin sensitivity also occurs after stomach cancer surgery, but before being affected by lifestyle changes. In this study, we evaluated the immediate improvement of glucose metabolism after gastrectomy for gastric cancer. In addition, we investigated whether these improvements were due to changes in insulin sensitivity or β-cell function.
Participants are patients who were diagnosed with early gastric cancer (EGC) and already had T2DM from April 2014 to March 2015 at the Kosin University Gospel hospital. All patients underwent routine surgery for gastric cancer. The present study approved by the institutional review board of the Kosin University Gospel Hospital, Republic of Korea (KUGH 2018-02-005-001).
Anthropometric measurements and blood sampling were conducted prior to operation and 12-weeks after surgery. Height and weight were obtained, and body mass index (BMI) was calculated by dividing patient weight (kg) by height squared (m2). Blood pressure was measured on the left arm with an automated blood pressure monitor. Blood sampling was done after more than eight hours fasting. Plasma glucose was measured by the hexokinase method, and total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and lipoprotein cholesterol (LDL-C) levels were measured via enzymatic procedures using AD2400 (Siemens, Germany). Triglyceride (TG) was measured by the GPO method using an AD2400 (Siemens, Germany). Insulin and C-peptide levels were analyzed with direct chemiluminescent method using an ADVIA Centaur XPT (Siemens, Germany).
All patients underwent Billroth-II (B-II) or total gastrectomy with Roux-en Y (R-Y). All patients received radical oncologic resection. Radical oncologic gastric resection includes omentectomy, lymph node dissection with vagotomy, and resection margin, which should be negative. The subtotal gastrectomy was performed, with approximately 75% of the distal stomach consisting of resection B-II reconstruction of intestinal continuation. In the case of B-II reconstruction, anastomosis was done at the site of 15 to 20 cm afferent limb. In the case of total gastrectomy, an R-Y esophagojejunostomy was performed with 40 cm of Roux limb and 20 cm of afferent limb.
All patients underwent a 75 g oral glucose tolerance test (OGTT) before and after gastrectomy. Post-surgical OGTT was done within one or two weeks after surgery. Glucose, insulin, and c-peptide were measured before, and then 30 and 120 minutes after 75 g glucose loading. OGTT was done after more than eight hours of overnight fasting. The area under the curve (AUC) of glucose was calculated before and after surgery. We performed a homeostasis model assessment of β-cell (HOMA-β)5 and insulinogenic index6 as an estimate of β-cell function. The HOMA-beta cell function (HOMA-β) was calculated by using the following formula: (20 . fasting insulin (μIU/mL)) / (fasting glucose (mg/dL) - 3.5). The insulinogenic index was calculated by using the following formula: (insulin at 30 mi-insulin at 0 min) / (glucose at 30 min-glucose at 0 min). Insulin sensitivity was estimated by homeostasis model assessment for insulin resistance (HOMA-IR)5 and Matsuda index.7 HOMA-IR was computed with the formula: fasting insulin (μIU/mL) . (fasting glucose (mg/dL) / 18) / 22.5. The Matsuda index was calculated using the following formula: 10,000/square root of [fasting glucose . fasting insulin] . [mean glucose . mean insulin during OGTT].
Statistical analysis was carried out with SPSS 19.0 (Chicago, IL). A paired t-test was used to compare the changes of anthropometric measurements, blood pressure, serum lipid levels, and indices of β-cell function and insulin sensitivity at baseline after surgery. All data are presented as the mean and standard deviation (SD). A P-value < 0.05 was considered statistically significant.
Go to : 
The mean patient age was 57.23 ± 11.08 years old. From a total of 14 patients, eight were male. Patients who had DM for more than five years numbered eight. One patient used insulin, eight patients used oral hypoglycemic agents, and five patients took no medication.
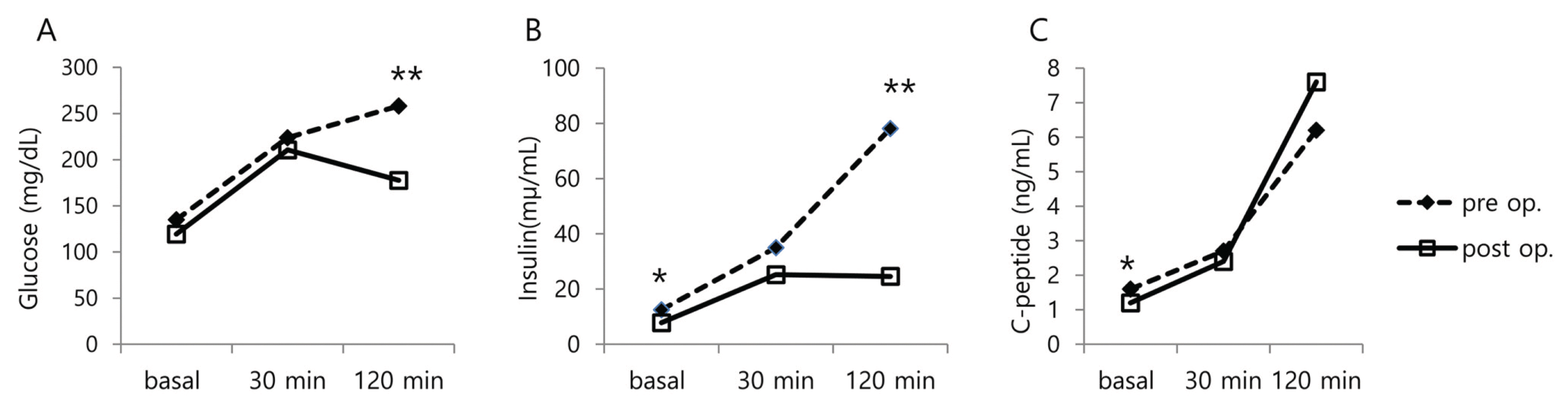
The results of OGTT before and within two weeks after surgery are represented in Figure 1. Plasma glucose and insulin levels at 120 minutes after glucose loading were significantly decreased after surgery (177.6 vs. 258.1 mg/dL, P = 0.001, 24.6 vs. 78.1 μU/mL, P = 0.001, respectively). C-peptide level at 12 minutes was increased after surgery, but not at a significant level (7.6 vs. 6.2 ng/mL, P = 0.747).
Table 1 shows the immediate changes of insulin sensitivity and beta cell function after gastrectomy. Fasting blood sugar deceased from 136.2 to 119.2 after surgery, but this difference is not statistically significant. HOMA-IR significantly decreased after surgery. HOMA-beta and insulinogenic index increased after surgery. The Matsuda index significantly increased, while AUC significantly decreased after surgery.
Immediate changes of insulin sensitivity and beta cell function after gastrectomy
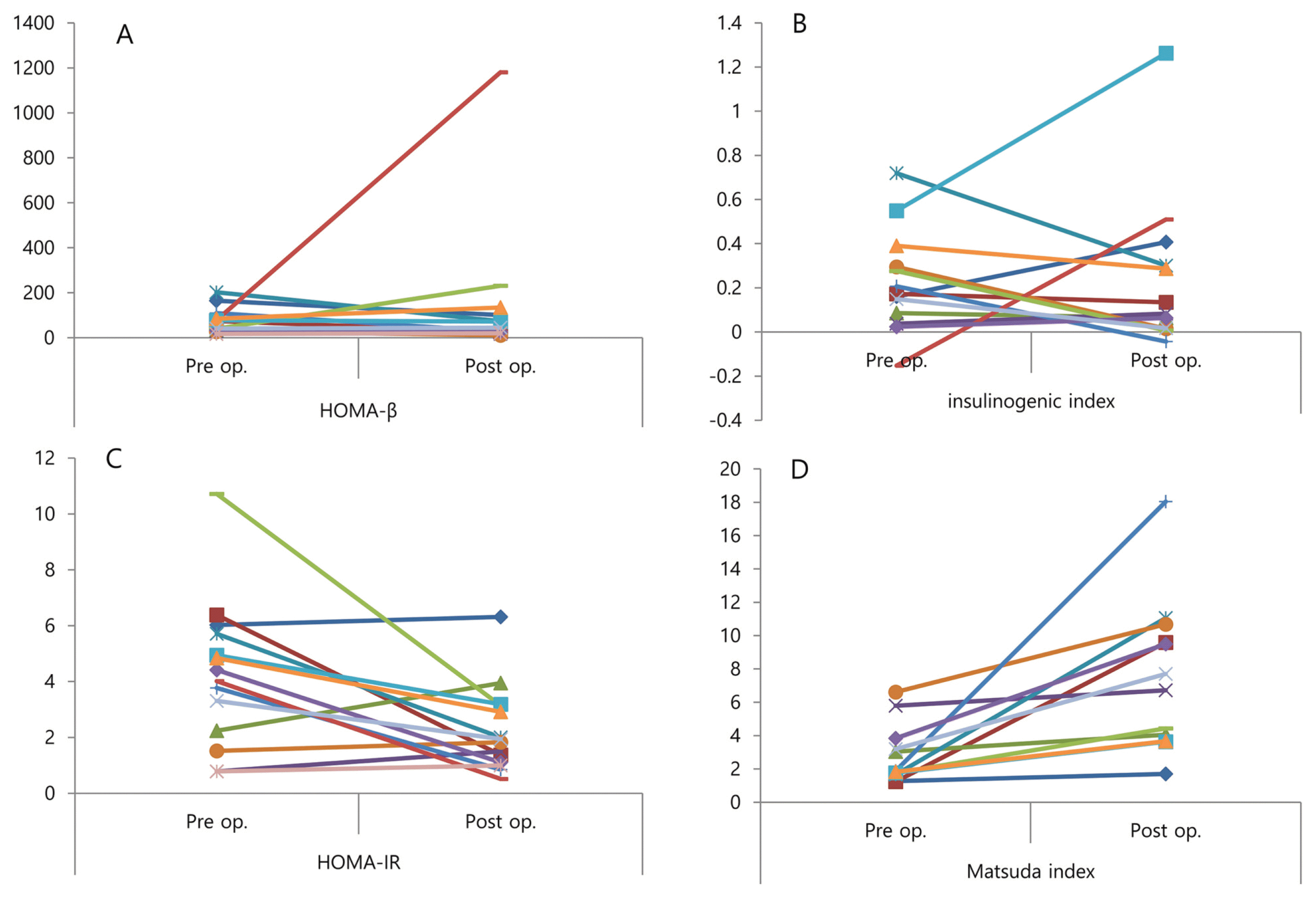
Figure 2. exhibits individual changes of beta cell function and insulin sensitivity before and within two weeks after surgery. HOMA-β and insulinogenic index changed in various directions after surgery. On the other hand, HOMA-IR and Matsuda index showed consistency between pre- to post-operation levels.
Although eight of nine patients reduced or stopped their anti-diabetic drugs after surgery, the mean HbA1c was reduced from 7.24% to 6.5% at three months after surgery. The changes of anthropometric measurements, blood pressure, and serum lipid levels before and three months after surgery are presented in Table 2. Weight and BMI values were significantly decreased after surgery.
Comparison between preoperative and postoperative (at 3 month after surgery)
Go to : 
Asian patients with stomach cancer have a normal body weight. In previous studies with stomach cancer patients, mean BMI values were 22–24 kg/m2 in Korea.1,8 In this study, the preoperative mean BMI was 28.4 ± 6.4 kg/m2, which is higher than previous studies; this is because this study included only EGC patients, and two patients had extremely high BMI. After gastric surgery, almost all patients showed weight loss.9 The participants of this study also demonstrated weight loss and their mean BMI decreased to 24.5 ± 14.4 kg/m2 three months after surgery. The improved diabetes symptoms in previous studies may be due to weight loss. We performed the oral glucose tolerance test within two weeks after surgery and before significant weight loss was observed. Further, we investigated the mechanism of improving blood glucose regardless of weight reduction.
After surgery, glucose level was significantly decreased at 120 minutes after OGTT. Insulin level was also significantly decreased after fasting and at the OGTT 120 minute time point. C-peptide was only significantly decreased upon fasting (Fig. 1). AUCglucose was also significantly reduced (from 512.9 ± 132.4 to 388.7 ± 78.5) after surgery. These results suggest that blood glucose level is improved immediately after surgery.
We also showed the improvement of glucose metabolism was not because improved β-cell function, but due to insulin resistance. In metabolic surgery, the most important change after surgery is dramatic improvement of diabetes symptoms. The main pathogenesis of type 2 diabetes is increasing insulin resistance and failure of insulin secretion from β-cells. Therefore, the improvement of diabetes means decreasing insulin resistance, increasing β-cell secretion, or both. Several studies revealed increased insulin secretion and β-cell function improvement after bariatric surgery.10,11 On the contrary, several studies proved the increasing β-cell function depends on the preoperative β-cell function.12–14 Our data also showed the representing factors of β-cell function did not improve (Table 1). HOMA-beta did not change individually after surgery, except in one patient (Fig. 2).
Improved insulin resistance has been demonstrated in many studies. In particular, some studies have shown that immediate improvement in insulin resistance is due to hepatic insulin resistance in the liver.15–17 In this study, the HOMA-IR and Matsuda index were significantly improved immediately after surgery; almost every patient showed improved HOMA-IR and Matsuda index (Fig. 2).
These improvements may be caused by calorie restriction, not by operation. Patients should fast for several days before and after surgery. Lingvay et al. suggested rapid improvement in diabetes symptoms after gastric bypass surgery due to calorie restriction itself.18 Nevertheless, postoperative diabetes improvement is not explained by only fasting. The improvement effect is maintained after beginning to eat. In this study, the improvement of blood glucose was maintained until three months after surgery.
A limitation of this study is the small number of patients evaluated. Prospective studies with a large number of patients may be needed. It also seems necessary to observe at long-term changes of insulin sensitivity. Patients may have had a healthier lifestyle or thorough blood sugar control after cancer diagnosis, as well as the effects of calorie restriction due to surgery. In this study, no investigation into changes in lifestyle after cancer diagnosis was conducted. These lifestyle changes can be expected to have a positive effect on insulin secretion and resistance. Another limitation is that we could not analyze differences by operation type. Nevertheless, this study is the first to analyze the immediate improvement of glucose metabolism after stomach cancer surgery. And the most reliable point of this study was the dynamic analysis of glucose metabolism by performing OGTT before and after surgery. So, this study could not just reveal changes in blood sugar level but could show the mechanism based on insulin resistance and beta cell function. In addition, only EGC patients were included, thus minimizing the impact of stomach cancer itself.
In conclusion, glucose metabolism was improved immediately after stomach cancer surgery in EGC patients. Insulin resistance was significantly improved, but β-cell function. The immediate change of glucose metabolism may be due to insulin resistance, even in early gastric cancer patients after gastrectomy. These results could be considered in the treatment of early gastric cancer patients with diabetes.
Go to : 
ACKNOWLEDGEMENTS
This study was supported by Kosin Society of Metabolic and Obesity Surgery (KOSMOS).
Go to : 
REFERENCES
1. Kim JW, Cheong JH, Hyung WJ, Choi SH, Noh SH. Outcome after gastrectomy in gastric cancer patients with type 2 diabetes. World J Gastroenterol. 2012; 18:49–54.

2. Kang KC, Shin SH, Lee YJ, Heo YS. Influence of gastrectomy for stomach cancer on type 2 diabetes mellitus for patients with a body mass index less than 30 kg/m(2). J Korean Surg Soc. 2012; 82:347–55.

3. Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995; 222:339–50. discussion 350–2.

4. Fried M, Ribaric G, Buchwald JN, Svacina S, Dolezalova K, Scopinaro N. Metabolic surgery for the treatment of type 2 diabetes in patients with BMI <35 kg/m2: an integrative review of early studies. Obes Surg. 2010; 20:776–90.
5. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985; 28:412–9.
6. Seino Y, Ikeda M, Yawata M, Imura H. The insulinogenic index in secondary diabetes. Hormone and Metabolic Research. 1975; 7(1):07–15.

7. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999; 22:1462–70.

8. Lee H-J, Kim H-H, Kim M-C, Ryu S-Y, Kim W, Song K-Y, et al. The impact of a high body mass index on laparoscopy assisted gastrectomy for gastric cancer. Surgical endoscopy. 2009; 23:2473–9.

9. Aoyama T, Yoshikawa T, Shirai J, Hayashi T, Yamada T, Tsuchida K, et al. Body weight loss after surgery is an independent risk factor for continuation of S-1 adjuvant chemotherapy for gastric cancer. Ann Surg Oncol. 2013; 20:2000–6.

10. Salehi M, Prigeon RL, D’Alessio DA. Gastric Bypass Surgery Enhances Glucagon- Like Peptide 1–Stimulated Postprandial Insulin Secretion in Humans. Diabetes. 2011; 60:230 8–14.
11. Anderwald C-H, Tura A, Promintzer-Schifferl M, Prager G, Stadler M, Ludvik B, et al. Alterations in gastrointestinal, endocrine, and metabolic processes after bariatric Roux-en-Y gastric bypass surgery. Diabetes care. 2012; 35:2580–7.

12. Jimenez A, Casamitjana R, Flores L, Delgado S, Lacy A, Vidal J. GLP-1 and the long-term outcome of type 2 diabetes mellitus after Roux-en-Y gastric bypass surgery in morbidly obese subjects. Ann Surg. 2013; 25(7):894–9.
13. Huang C-K, Shabbir A, Lo C-H, Tai C-M, Chen Y-S, Houng J-Y. Laparoscopic Roux-en-Y gastric bypass for the treatment of type II diabetes mellitus in Chinese patients with body mass index of 25–35. Obes surg. 2011; 21:1344–9.

14. Malapan K, Goel R, Tai C-M, Kao Y-H, Chang P-C, Huang C-K. Laparoscopic Roux-en-Y gastric bypass for nonobese type II diabetes mellitus in Asian patients. Surg Obes Relat Dis. 2014; 10:834–40.

15. Bojsen-Moller KN, Dirksen C, Jorgensen NB, Jacobsen SH, Serup AK, Albers PH, et al. Early enhancements of hepatic and later of peripheral insulin sensitivity combined with increased postprandial insulin secretion contribute to improved glycemic control after Roux-en-Y gastric bypass. Diabetes. 2014; 63:1725–37.

16. Salinari S, Bertuzzi A, Asnaghi S, Guidone C, Manco M, Mingrone G. First-phase insulin secretion restoration and differential response to glucose load depending on the route of administration in type 2 diabetic subjects after bariatric surgery. Diabetes Care. 2009; 32:375–80.

17. Bojsen-Moller KN, Dirksen C, Jorgensen NB, Jacobsen SH, Hansen DL, Worm D, et al. Increased hepatic insulin clearance after Roux-en-Y gastric bypass. J Clin Endocrinol Metab. 2013; 98:E1066–71.
18. Lingvay I, Guth E, Islam A, Livingston E. Rapid improvement in diabetes after gastric bypass surgery : is it the diet or surgery? Diabetes care. 2013; 36:2741–7.
Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download