MATERIALS AND METHODS
Animals
Drugs
Formalin-induced nociceptive behavioral test
DRGs and spinal cords were harvested from the right lumbar segments
Immunohistochemistry
Statistical analysis
RESULTS
Formalin-induced nociceptive behavioral test
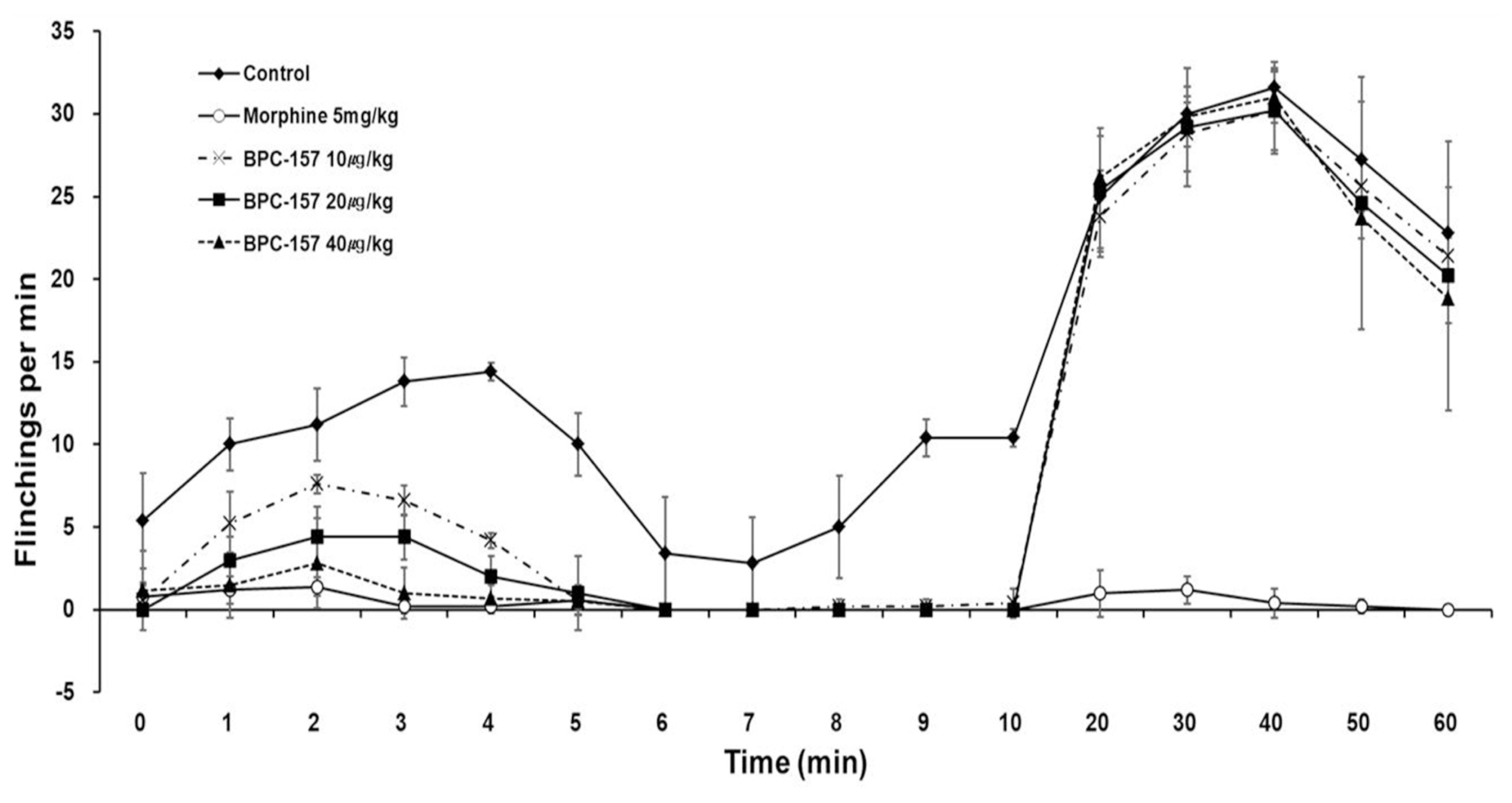 | Fig. 1Time-effect curve of the control, morphine, and BPC-157 (from 10 to 40 μg/kg) groups of the flinching response in the formalin testData are presented as the number of flinching and licking/biting. BPC-157 significantly suppressed flinches in a dose-dependent manner in phase 1. Each line represents the mean and standard error bars of 7 rats.
|
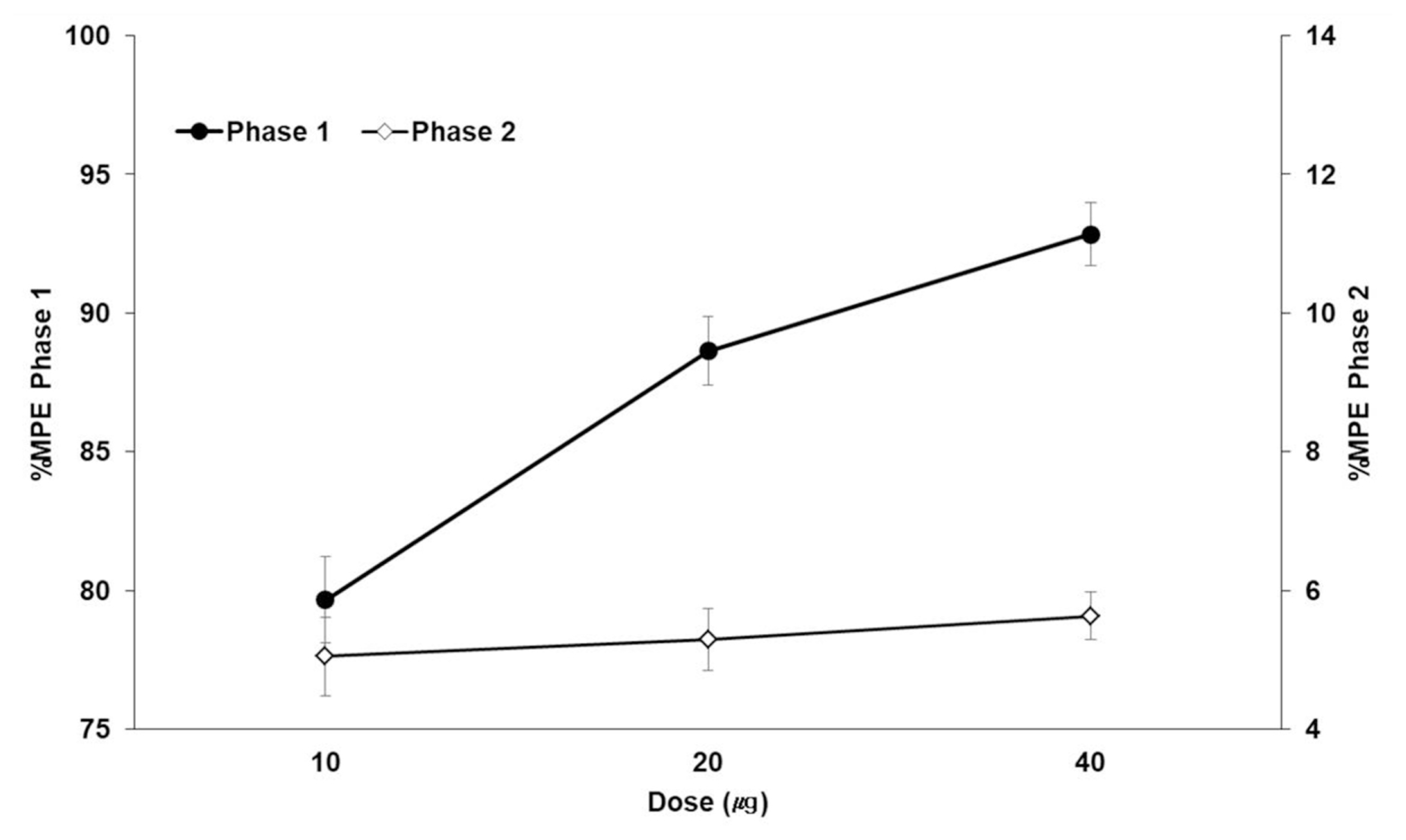 | Fig. 2Dose-response curve of the flinching response after intraperitoneal BPC-157 administration in the formalin testData are presented as the percentage of control. Pretreatment with BPC-157 significantly suppressed flinches in a dose-dependent manner in phase 1 but showed little effect in phase 2. Each line represents the mean % MPE (% maximal possible effect) and standard error bars of 7 rats.
|
Immunohistochemistry for cytokines
 | Fig. 3Immunohistochemistry of interleukin-1β (IL-1β) in the dorsal root ganglion (DRG) and spinal cord (SC) at (A) 5 min (representative value of phase 1) and (B) 35 min (representative value of phase 2)Graph (bottom) represents the number of immunostained cells in the DRG and SC (mean ± SD). (A): Significant differences was achieved in the DRG tissues from the morphine and sham groups (#, P < 0.001) and from the 10 and 20 μg/kg BPC-157 groups (*, P < 0.05) compared those from the control group. The SC tissues of the morphine and sham groups showed significant differences from those of the control group (*, P < 0.05). (B): Statistical significance was achieved in the DRG tissues from the morphine and sham groups (#, P < 0.001) and the 20 and 40 μg/kg BPC-157 groups (*, P < 0.05) compared those from the control group. The SC tissues of the morphine and sham groups showed significant differences from those of the control group (*, P < 0.05).
|
 | Fig. 4Immunohistochemistry of interleukin-6 (IL-6) in the dorsal root ganglion (DRG) and spinal cord (SC) at phases (A) 1 and (B) 2Graph (bottom) represents the number of immunostained cells in the DRG and SC (mean ± SD). (A): Statistical significance was achieved in the DRG tissues from the morphine and sham groups (#, P < 0.001) and the BPC-157 10 μg/kg group (*, P < 0.05) compared with those from the control group. (B): Statistical significance was achieved in both the DRG with SC tissues from the morphine and sham groups (#, P < 0.001) and the 20 μg/kg BPC-157 group (*, P < 0.05) compared with those from the control group.
|
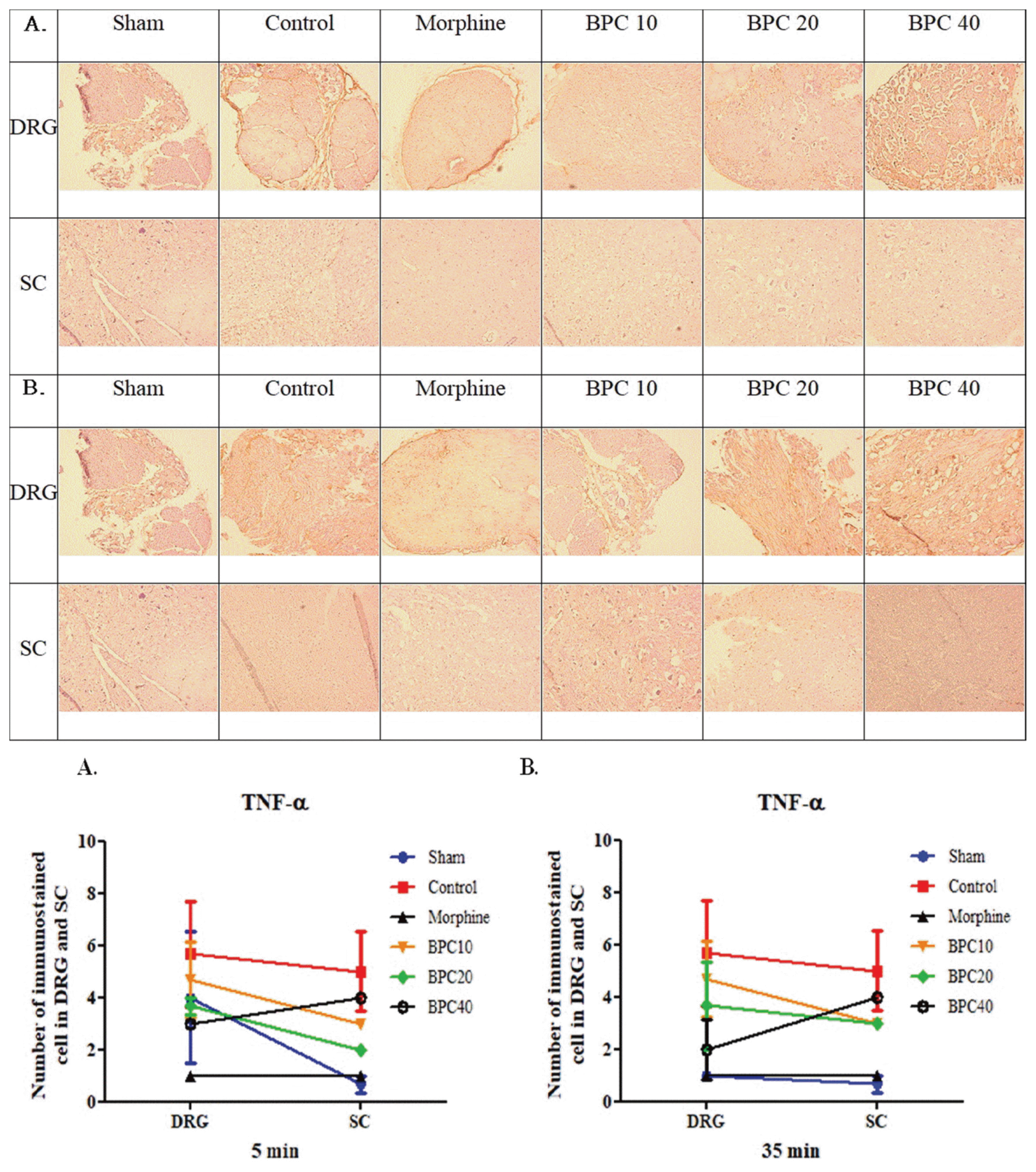 | Fig. 5Immunohistochemistry of tumor necrosis factor-α (TNF-α) in the dorsal root ganglion (DRG) and spinal cord (SC) at phases (A) 1 and (B) 2Graph (bottom) represents the number of immunostained cells in the DRG and SC (mean ± SD). There were no differences between the control and experimental groups during either phase.
|




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download