1. Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998; 98:946–952. PMID:
9737513.
2. Verma A, Marrouche NF, Natale A. Pulmonary vein antrum isolation: intracardiac echocardiography-guided technique. J Cardiovasc Electrophysiol. 2004; 15:1335–1340. PMID:
15574190.
3. Charitos EI, Stierle U, Ziegler PD, et al. A comprehensive evaluation of rhythm monitoring strategies for the detection of atrial fibrillation recurrence: insights from 647 continuously monitored patients and implications for monitoring after therapeutic interventions. Circulation. 2012; 126:806–814. PMID:
22824434.
4. Martinek M, Aichinger J, Nesser HJ, Ziegler PD, Purerfellner H. New insights into long-term follow-up of atrial fibrillation ablation: full disclosure by an implantable pacemaker device. J Cardiovasc Electrophysiol. 2007; 18:818–823. PMID:
17573835.

5. Ziegler PD, Koehler JL, Mehra R. Comparison of continuous versus intermittent monitoring of atrial arrhythmias. Heart Rhythm. 2006; 3:1445–1452. PMID:
17161787.

6. Hindricks G, Pokushalov E, Urban L, et al. Performance of a new leadless implantable cardiac monitor in detecting and quantifying atrial fibrillation: results of the XPECT trial. Circ Arrhythm Electrophysiol. 2010; 3:141–147. PMID:
20160169.

7. Passman RS, Weinberg KM, Freher M, Schaechter A, Goldberger JJ, Kadish AH. Accuracy of mode switch algorithms for detection of atrial tachyarrhythmias. J Cardiovasc Electrophysiol. 2004; 15:773–777. PMID:
15250860.

8. Charitos EI, Stierle U, Ziegler PD, et al. A comprehensive evaluation of rhythm monitoring strategies for the detection of atrial fibrillation recurrence: insights from 647 continuously monitored patients and implications for monitoring after therapeutic interventions. Circulation. 2012; 126:806–814. PMID:
22824434.
9. Kottkamp H, Tanner H, Kobza R, et al. Time courses and quantitative analysis of atrial fibrillation episode number and duration after circular plus linear left atrial lesions: trigger elimination or substrate modification: early or delayed cure? J Am Coll Cardiol. 2004; 44:869–877. PMID:
15312874.
10. Verma A, Champagne J, Sapp J, et al. Discerning the incidence of symptomatic and asymptomatic episodes of atrial fibrillation before and after catheter ablation (DISCERN AF): a prospective, multicenter study. JAMA Intern Med. 2013; 173:149–156. PMID:
23266597.
11. Andrade JG, Champagne J, Dubuc M, et al. Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring: a randomized clinical trial. Circulation. 2019; 140:1779–1788. PMID:
31630538.
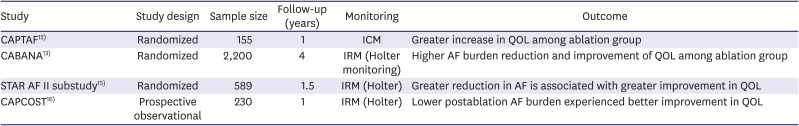
12. Blomström-Lundqvist C, Gizurarson S, Schwieler J, et al. Effect of catheter ablation vs antiarrhythmic medication on quality of life in patients with atrial fibrillation: the CAPTAF randomized clinical trial. JAMA. 2019; 321:1059–1068. PMID:
30874754.
13. Packer DL, Mark DB, Robb RA, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019; 321:1261–1274. PMID:
30874766.
14. Mantovan R, Macle L, De Martino G, et al. Relationship of quality of life with procedural success of atrial fibrillation (AF) ablation and postablation AF burden: substudy of the STAR AF randomized trial. Can J Cardiol. 2013; 29:1211–1217. PMID:
23988341.

15. Verma A, Sanders P, Macle L, et al. Substrate and Trigger Ablation for Reduction of Atrial Fibrillation Trial-Part II (STAR AF II): design and rationale. Am Heart J. 2012; 164:1–6.e6. PMID:
22795275.

16. Terricabras M, Mantovan R, Jiang CY, et al. Association between quality of life and procedural outcome after catheter ablation for atrial fibrillation: a secondary analysis of a randomized clinical trial. JAMA Netw Open. 2020; 3:e2025473. PMID:
33275151.
17. Essebag V, Azizi Z, Alipour P, et al. Relationship between quality of life and burden of recurrent atrial fibrillation following ablation: CAPCOST multicentre cohort study. Europace. 2020; 22:1017–1025. PMID:
32531030.

18. Piccini JP, Passman R, Turakhia M, Connolly AT, Nabutovsky Y, Varma N. Atrial fibrillation burden, progression, and the risk of death: a case-crossover analysis in patients with cardiac implantable electronic devices. Europace. 2019; 21:404–413. PMID:
30462208.

19. Wong JA, Conen D, Van Gelder IC, et al. Progression of device-detected subclinical atrial fibrillation and the risk of heart failure. J Am Coll Cardiol. 2018; 71:2603–2611. PMID:
29880119.
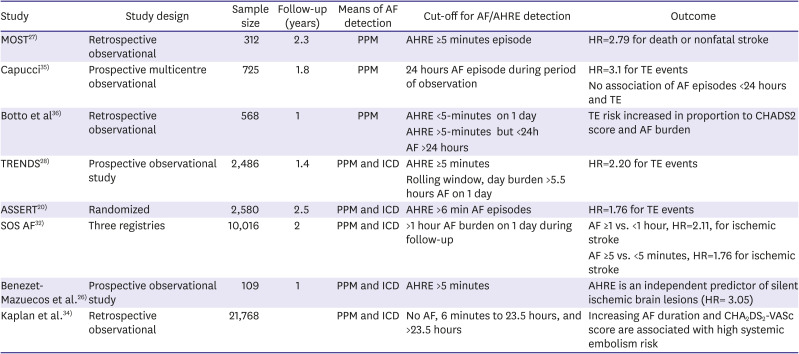
20. Hohnloser SH, Capucci A, Fain E, et al. ASymptomatic atrial fibrillation and Stroke Evaluation in pacemaker patients and the atrial fibrillation Reduction atrial pacing Trial (ASSERT). Am Heart J. 2006; 152:442–447. PMID:
16923410.
21. Gonzalez M, Keating RJ, Markowitz SM, et al. Newly detected atrial high rate episodes predict long-term mortality outcomes in patients with permanent pacemakers. Heart Rhythm. 2014; 11:2214–2221. PMID:
25131667.

22. Ganesan AN, Chew DP, Hartshorne T, et al. The impact of atrial fibrillation type on the risk of thromboembolism, mortality, and bleeding: a systematic review and meta-analysis. Eur Heart J. 2016; 37:1591–1602. PMID:
26888184.

23. Ali AN, Abdelhafiz A. Clinical and economic implications of AF related stroke. J Atr Fibrillation. 2016; 8:1279. PMID:
27909470.
24. Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace. 2018; 20:e1–160.
25. Hindricks G, Piorkowski C, Tanner H, et al. Perception of atrial fibrillation before and after radiofrequency catheter ablation: relevance of asymptomatic arrhythmia recurrence. Circulation. 2005; 112:307–313. PMID:
16009793.

26. Benezet-Mazuecos J, Rubio JM, Cortés M, et al. Silent ischaemic brain lesions related to atrial high rate episodes in patients with cardiac implantable electronic devices. Europace. 2015; 17:364–369. PMID:
25336664.

27. Glotzer TV, Hellkamp AS, Zimmerman J, et al. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST). Circulation. 2003; 107:1614–1619. PMID:
12668495.
28. Glotzer TV, Daoud EG, Wyse DG, et al. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol. 2009; 2:474–480. PMID:
19843914.
29. Van Gelder IC, Healey JS, Crijns HJ, et al. Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J. 2017; 38:1339–1344. PMID:
28329139.

30. Gage BF, van Walraven C, Pearce L, et al. Selecting patients with atrial fibrillation for anticoagulation: stroke risk stratification in patients taking aspirin. Circulation. 2004; 110:2287–2292. PMID:
15477396.
31. Stroke Risk in Atrial Fibrillation Working Group. Comparison of 12 risk stratification schemes to predict stroke in patients with nonvalvular atrial fibrillation. Stroke. 2008; 39:1901–1910. PMID:
18420954.
32. Boriani G, Glotzer TV, Santini M, et al. Device-detected atrial fibrillation and risk for stroke: an analysis of >10,000 patients from the SOS AF project (Stroke preventiOn Strategies based on Atrial Fibrillation information from implanted devices). Eur Heart J. 2014; 35:508–516. PMID:
24334432.
33. Turakhia MP, Ziegler PD, Schmitt SK, et al. Atrial fibrillation burden and short-term risk of stroke: case-crossover analysis of continuously recorded heart rhythm from cardiac electronic implanted devices. Circ Arrhythm Electrophysiol. 2015; 8:1040–1047. PMID:
26175528.
34. Kaplan RM, Koehler J, Ziegler PD, Sarkar S, Zweibel S, Passman RS. Stroke risk as a function of atrial fibrillation duration and CHA
2DS
2-VASc score. Circulation. 2019; 140:1639–1646. PMID:
31564126.
35. Capucci A, Santini M, Padeletti L, et al. Monitored atrial fibrillation duration predicts arterial embolic events in patients suffering from bradycardia and atrial fibrillation implanted with antitachycardia pacemakers. J Am Coll Cardiol. 2005; 46:1913–1920. PMID:
16286180.

36. Botto GL, Padeletti L, Santini M, et al. Presence and duration of atrial fibrillation detected by continuous monitoring: crucial implications for the risk of thromboembolic events. J Cardiovasc Electrophysiol. 2009; 20:241–248. PMID:
19175849.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download