INTRODUCTION
Mechanical circulatory support (MCS) is designed to provide blood flow to vital organs in patients with conditions that impair end-organ perfusion. Such conditions include but are not limited to acute myocardial infarction complicated by cardiogenic shock, high-risk percutaneous coronary intervention (PCI), and end-stage congestive heart failure (CHF). While many MCS devices do increase blood pressure and both coronary and peripheral organ perfusion, they carry risks such as hemolysis, vascular complications, and stroke. Randomized trials of MCS have been challenging to complete, and those that have been completed have shown mixed results.
1) On the other hand, there are several large observational analyses (i.e., “real-world” data) examining the association between MCS use and outcomes. Some of these have focused on the intravascular microaxial left ventricular flow pump, the Impella (Abiomed, Danvers, MA, USA), or the intra-aortic balloon pump (IABP), and have studied the comparative effectiveness of the Impella against IABP. The purpose of this narrative review is to summarize the observational “real-world” data comparing the association between Impella or IABP and clinical outcomes and assess the limitations of such data. Of note, this review is limited to multicenter observational studies. Single center reports and abstract presentations were not considered.
HIGH-RISK PERCUTANEOUS CORONARY INTERVENTION
One challenge with performing observational analyses of high-risk PCI procedures is determining what constitutes “high-risk.” Many registries do not adequately capture enough angiographic detail to assess the relationship between vessel and lesion characteristics and outcome. While there are data showing that angiographic variables do not appreciably increase the risk for mortality,
2) some likely influence the risk of acute procedural success and clinical outcome. For example, chronic total occlusions (CTO) carry a greater risk for procedural failure and complications compared with non-CTO lesions; however, there is heterogeneity in the complexity of CTOs
3) that may not be captured in registry datasets. In addition to angiographic variables, clinical features like CHF, cardiogenic shock, or low ejection fraction can influence outcomes.
4) These limitations must be considered when assessing observational data on the use of MCS.
There have been several real-world studies of Impella in high-risk PCI that are single center and do not include a control group. A systematic overview of Impella studies was performed by Ait Ichou and colleagues
5) examining published papers through 2016. After excluding studies with fewer than 10 patients, commentaries, meta-analyses, review articles, editorials, cross-sectional studies, and letters to the editor, they found 20 papers. Of these, 4 were randomized trials, 3 of which had a total of 49 Impella treated patients combined. The remainder were observational studies, and only 2 of those included a control group. Many of the studies from multicenter registries performed in the US and Europe had sample sizes less than 100 and showed that patients undergoing PCI with Impella have characteristics that would be considered high-risk for mortality. For example, the rate of diabetes among enrolled patients in the USPella registry undergoing high-risk PCI was 47% and the baseline mean cardiac output was 2.1 L/min.
6) Cohen and colleagues compared characteristics of patients undergoing high-risk PCI with Impella support from the USPella registry with outcomes from the PROTECT II randomized trial.
7) The PROTECT II trial was a randomized trial of high-risk PCI comparing the Impella 2.5 device with IABP.
1) The primary endpoint of 30-day major adverse cardiovascular event rates at 30 days were not statistically different between the 2 devices (35.1% Impella vs. 40.1% IABP, p=0.227), but trended in favor of Impella 2.5 at 90 days. In the analysis by Cohen, registry patients were older, had more medical comorbid conditions, and more extensive coronary artery disease compared with patients in the PROTECT II trial. In-hospital mortality was non-significantly higher among registry patients compared with trial participants (4.6% vs. 2.7%, p=0.27). Rates of 30-day all-cause mortality across the other smaller studies range from 0–42.6% and 30-day major adverse cardiac event rates range from 0–20%. Given the small sample sizes of some of these studies and lack of a control group, it is difficult to draw any firm conclusions about the relative efficacy of Impella compared with other MCS. However, the greater hemodynamic support afforded by the Impella could confer an advantage over IABP. The PROTECT IV randomized trial that is comparing the Impella CP or Impella 2.5 with standard of care PCI with or without IABP will provide valuable data on clinical outcomes between these 2 devices (
https://clinicaltrials.gov/ct2/show/NCT04763200?term=Impella&draw=2&rank=5).
Administrative databases allow for a multicenter assessment of MCS use and comparative effectiveness of different MCS devices. One available dataset is the National Inpatient Sample, which is a payer database of a random 20% of the inpatients treated in the United States. Using these data, Khera et al.
8) examined trends in the use of Impella between 2007 and 2012. Both Impella and the TandemHeart device (Tandem Life, Pittsburgh, PA, USA) were grouped together as percutaneous ventricular assist devices (PVADs). Over the 6-year period, there was a 30-fold increase in PVAD use and a decrease in the use of IABP. PVADs were used more often among patients undergoing PCI rather than coronary artery bypass grafting and were used less often among those with cardiogenic shock. The authors constructed a propensity score based on propensity to receive PVAD or IABP, and matched 1,446 PVAD patients with 2,888 patients who received IABP. The propensity score matched analysis showed that the use of PVADs was associated with an increased risk of in-hospital mortality (odds ratio, 1.23; 95% confidence interval, 1.06–1.43).
An updated analysis of the National Inpatient Sample using data from 2012–2016 was performed by Philipson and colleagues.
9) The authors also examined reports submitted between 2008–2019 to the US Food and Drug Administration's Manufacturer and User Facility Device Experience (MAUDE) database, which is a database of voluntary reports of adverse events experienced by users of devices during post-market surveillance. They found that over the 11-year period, 885 reports were submitted to the MAUDE database reporting 1,206 complications related to Impella. The 3 most common reported complications were bleeding, deployment or retrieval issues, and vascular complications. Patient death was included in 12.4% of the submitted reports. Importantly, 7.9% of reported complications were deemed attributable to operator decision-making or technique, underscoring the importance of experience and proficiency with Impella in reducing complications. Analysis of the National Inpatient Sample data showed that the use of Impella continued to increase between 2012 and 2017. Unadjusted mortality among inpatients receiving Impella increased from 23.1% in 2009 to 31% between 2012–2016, and a slight decrease to 28.6% in 2017.
The challenge with administrative data is that it may lack sufficient clinical detail to partly account for selection bias. Other databases may contain more clinical covariates that can be included in adjusted analyses. For example, Amin and colleagues used the Premier database to compare the association between Impella use and outcomes compared with IABP among patients undergoing PCI.
10) The dataset consisted of over 1.7 million PCI procedures from 432 hospitals. Between 2008 and 2016, the use of MCS increased from 2.5% to 3.5%, with Impella accounting largely for the increase. Among patients undergoing PCI with MCS, the use of Impella increased to 31.9% of cases. There was a concomitant decline in the use of IABP and significant variation in the use of MCS across hospitals. There was an imbalance in baseline patient and procedural characteristics between those who received IABP vs. those who received Impella such that the latter was used more often in patients with diabetes mellitus, chronic obstructive pulmonary disease, and multivessel coronary artery disease. In contrast, IABP was used more often in patients with ST-segment elevation myocardial infarction and those presenting in cardiogenic shock. When hospitals with higher Impella use were compared with those that had lower use, there was a higher risk of death, bleeding, acute kidney injury, and stroke among higher use hospitals that persisted after adjustment for propensity to receive Impella and clustering of patients across hospitals. At the patient level, Impella use was associated with a significantly increased adjusted risk for the individual outcomes of death, bleeding, and stroke compared with IABP (
Figure 1). The findings were consistent across several sensitivity analyses; the use of falsification endpoints indicated that the increased risk with Impella was less likely to be due to unmeasured comorbidities.
Figure 1
Association between Impella and clinical outcomes compared with intra-aortic balloon pump among patients undergoing percutaneous coronary intervention (reprinted from Amin et al.10)).
AKI = acute kidney injury; CI = confidence interval; OR = odds ratio.

CARDIOGENIC SHOCK
An important aspect of the studies summarized above is that they included patients undergoing PCI regardless of indication or procedure status. In these studies patients with cardiogenic shock are mixed with patients without shock, and patients undergoing PCI for acute myocardial infarction are mixed with those undergoing PCI for other indications. For example, in the National Inpatient Sample analyses, 42–67% of the included patients were coded as having cardiogenic shock. The largest real-world study focusing on Impella use in the setting of PCI for cardiogenic shock is from the American College of Cardiology National Cardiovascular Registry. The CathPCI registry, an ongoing contemporary database of PCI procedures, collects detailed procedural data and in-hospital outcomes from thousands of cardiac catheterization laboratories across the United States. Dhruva and colleagues examined 28,304 patients undergoing PCI for cardiogenic shock between 2015 and 2017.
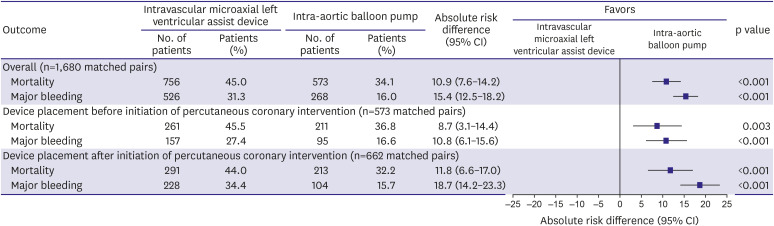
11) In this cohort, 57.3% received no MCS, 29.9% received an IABP, and 6.2% received an Impella. Propensity matching resulted in 1,680 well-matched pairs. In the propensity matched cohort, Impella use was associated with an increased risk of in-hospital mortality or major bleeding compared with IABP, regardless of whether the device was placed before or after the start of PCI (
Figure 2). Interestingly, the authors also performed a similar analysis comparing IABP with medical therapy among patients with cardiogenic shock undergoing PCI. They found a statistically significant increased risk of in-hospital mortality (IABP 28.6% vs. medical therapy alone 26.5%, p=0.002) and bleeding (IABP 14.5% vs. medical therapy alone 11.0%, p<0.001) associated with IABP use.
Figure 2
In-hospital outcomes among propensity matched patients with acute myocardial infarction and cardiogenic shock undergoing percutaneous coronary intervention with either Impella or intra-aortic balloon pump (reprinted from Dhruva et al.11)).
CI = confidence interval.
HOSPITAL COSTS OF MECHANICAL CIRCULATORY SUPPORT USE
Two studies have examined the association between Impella us and hospital costs. Using the National Inpatient Sample, Khera et al.
8) showed that the hospital costs for Impella treated patients amounted to a mean of $85,580, with higher mean costs among those with cardiogenic shock. The corresponding mean costs among IABP treated patients was $55,168. Similarly, Amin examined hospital costs of MCS use in 2 ways.
10) First, they compared costs in the “pre-Impella era” (2004–2009) and the “post-Impella era” (2010–2016). They found that adjusted costs of PCI hospitalizations were stable in the pre-Impella era but increased in the post-Impella era. Costs among PCI hospitalizations without MCS use declined, while costs among PCI hospitalizations with MCS increased (mean per-patient hospitalization cost increase of $1,775). Second, they compared costs between low Impella use hospitals and high Impella use hospitals in the Premier dataset. Compared with low-use hospitals (defined as 0% Impella use), increase Impella use was associated with a stepwise increase in hospitalization costs (
Table 1).
Table 1
Hospitalization costs of patients undergoing PCI with Impella support categorized by hospital Impella use

|
Impella use |
p value |
|
0% |
>0% to ≤3.33% |
>3.33% to ≤14.72% |
>14.72% |
|
Reference |
$11,002 ($6,987–$15,018) |
$12,039 ($10,770–$13,307) |
$12,071 ($11,067–$13,075) |
<0.001 |
LIMITATIONS OF OBSERVATIONAL STUDIES
Taken together, these real-world studies indicate that Impella is used in higher risk patients compared with the randomized PROTECT II trial, and administrative data show an association between Impella use and an increased risk for in-hospital adverse outcomes and costs compared with IABP among patients undergoing PCI with or without shock. On the other hand, it is important to underscore that the studies described are all observational; thus, causality should not be inferred. Observational studies can suffer from a number of confounding issues. Foremost of these is selection bias wherein sicker patients may have been selected for receiving Impella. In the study by Amin and colleagues,
10) patients who received Impella had some clinical features that were higher risk than those who received IABP, but they also were less often on mechanical ventilatory support, and less often had ST-segment elevation myocardial infarction or shock. Patients who may have been escalated from IABP to Impella were excluded from the analysis as well. In addition, there may be unmeasured confounding that drove the decision to use Impella instead of IABP in these patients. One of the most common types of confounding in analyses like those summarized above is confounding by indication, wherein the association between an exposure (e.g., Impella) and the outcome (e.g., mortality) is distorted by the presence of an indication for the exposure that is the true cause of the outcome (e.g., shock). This type of confounding is unlikely to be overcome using observational data. Real-world studies underscore the importance of adequately powered prospective randomized trials to guide the use of MCS in patients undergoing PCI.
FUTURE DIRECTIONS
While real-world studies of therapies are valuable for demonstrating patterns of use, performing comparative effectiveness analyses are limited by measured and unmeasured confounding. Particularly in the case of MCS, confounding by indication may hinder the application of the study to clinical practice. Prospective randomized trials are the standard by which the safety and efficacy of MCS should be judged. It has been challenging to complete such trials; in particular, trials involving MCS have several challenges that can be difficult to overcome, including lack of equipoise, physician preferences and proficiency, and difficulty obtaining informed consent (particularly in patients with shock).
12) Despite these issues, clinicaltrials.gov lists several ongoing registries and clinical trials involving Impella (
https://clinicaltrials.gov/ct2/results?term=Impella&Search=Apply&recrs=b&recrs=a&recrs=f&recrs=d&age_v=&gndr=&type=&rslt=). While it remains to be seen whether these studies will complete enrollment, they are likely to be informative once completed. Until the results of these trials are available, the use of MCS and Impella should be guided by available practice guidance statements (
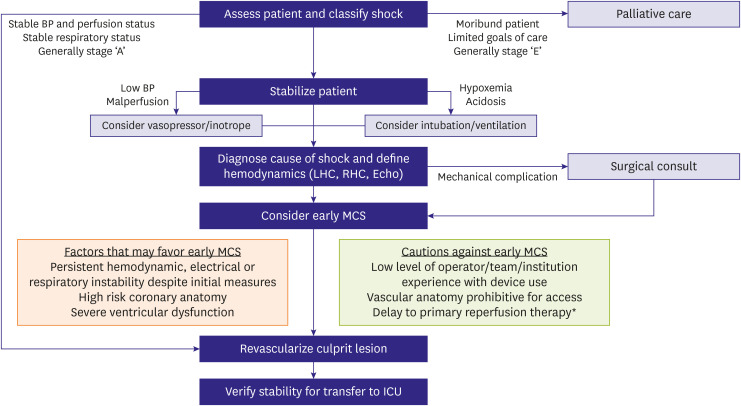
Figure 3).
13) The current American Heart Association scientific statement on the invasive management of cardiogenic shock recommends early institution of MCS in patients with persistent hemodynamic, electrical, or respiratory instability despite initial measures, high-risk coronary anatomy (e.g., last remaining vessel), or severe left ventricular dysfunction, but it acknowledges that more data are needed.
Figure 3
Recommended treatment algorithm for patients with cardiogenic shock from the American Heart Association scientific statement on the invasive management of acute myocardial infarction complicated by cardiogenic shock (reprinted from Henry et al.13)).
BP = blood pressure; ICU = intensive care unit; LHC = left heart catheterization; MCS = mechanical circulatory support; RHC = right heart catheterization.
CONCLUSIONS
While MCS can provide hemodynamic support for patients undergoing high-risk PCI and those with cardiogenic shock, they are also associated with significant risks such as vascular injury, major bleeding, and stroke. Randomized trials of MCS have been challenging to complete due to lack of equipoise and challenges with informed consent. The available real-world data show an association between the use of MCS and an increased risk for adverse outcomes, but these observational data are significantly limited by selection bias and confounding by indication. Randomized trials are ongoing and if they are able to reach completion, will inform clinical practice and practice guidelines.







 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download