Abstract
In February 2018, the Withdrawal of the Life-sustaining Treatment (WLST) Decision Act was legalized in Korea. Donation after circulatory death (DCD) after WLST was classified as DCD category III. We report the first case of successful organ donation after WLST in Korea. A 52-year-old male who experienced cerebral hemorrhage was a potential brain-dead donor with donation consent. During the first brain death examination, Babinski reflex was present, which disappeared two days later. Then, electroencephalography was performed five times at intervals of 2 to 3 days, according to the recommendation of a neurologist. The patient was transferred to the OR at 19:30 July 3, 2020. At 20:00, an intensive care unit specialist performed extubation and discontinued vasopressors. Oxygen saturation fell to < 70% in 1 minute, which signaled the beginning of functional warm ischemia. At 20:15, asystole was confirmed; after 5 minutes of “no-touch time,” circulatory death was declared. Organ procurement surgery was initiated, with surgeons performing the recipient surgery ready in the adjacent OR. Through the first successful DCD case, we expected that DCD will be actively implemented in Korea, saving the lives of patient waiting for transplantation and resolving the imbalance between organ receipt and donation.
Organ transplantation is an effective treatment method that prolongs the life of patients experiencing terminal organ failure and improves their quality of life.1 There are two types of organ donation: living and cadaveric. Cadaveric donation is based on the “dead donor rule,” and organs from donors who have been declared dead can always be donated.2 For this reason, the concept of brain death was introduced and organ donation after brain death (DBD) was enabled; however, the number of brain-dead donors is very small compared to the rapid increase in the number of individuals waiting for organ transplantation.3 Therefore, in an attempt to increase the number of donors, donation after circulatory death (DCD) has been proposed as an option.1
In many European countries, including Spain, the United Kingdom, and the United States, the concept of DCD was developed between 1980 and 2000; according to a study published in 2019, DCD has been enforced in 18 of 37 European countries.3 DCD is being implemented in many countries, leading to an increase in the number of DCDs among the total donors (Spain, 28%; United States, 19.9%; and United Kingdom, 38%) since 2018.4
In Korea, only DBD and transplantation are authorized. As of December 2019, the number of individuals waiting for solid organ transplantation was > 40,000, while the number of potential brain-dead donors was only 450, significantly lower than the number of individuals waiting for transplant.5 Due to these long-term imbalances between supply and demand, the interest in DCD rose in Korea, with a total of 34 DCDs performed between 2006 and 2015. These cases were all cardiac arrests that occurred during the process of determining brain death; in Korea, DCD is performed in a limited manner within Maastricht category IV.6
Regarding the status of implementation of the withdrawal of life-sustaining treatment (WLST) after the enforcement of the Life-Prolonging Medical Care Decision Law (February 2018) in the domestic field of life-prolonging medicine, the number of individuals in the base month rapidly increased to 30,000 in February 2019. Among these, 26.5% were patients with respiratory, heart, and brain diseases.7 If families wish to donate the organs of these patients, the possibility of DCD after discontinuation of life-sustaining medical care can be predicted.
If the recognition of discontinuation of life-sustaining medical care and organ donation is a process that can be selected in the end-of-life process of individuals, domestic DCD can be activated. Herein, we report Korea's first DCD case after WLST after failure of brain death determination during the process of brain-dead donor management at Korea University Anam Hospital, Seoul, Republic of Korea.
A 52-year-old male was found in the bathroom where he had collapsed while taking a shower. He was diagnosed with cerebral hemorrhage using computed tomography performed in the emergency room, and extra-ventricular drainage was performed. Subsequently, emergency craniectomy was put forth as an option. However, after being informed about the poor prognosis and high probability of death after surgery, the patient's wife signed a memorandum to forgo the operation. On day 2 of hospitalization, brain death was presumed during conservative treatment in the intensive care unit (ICU), and the possibility of organ donation was explained to the family, who agreed.
During the first brain death investigation performed by a neurologist, the Babinski reflex was present; as such, the investigation was discontinued. On day 4 of hospitalization, the Babinski reflex was absent, and a criterion for the first brain death investigation performed by a neurosurgeon was fulfilled.
Thereafter, the Korean Network of Organ Sharing (KONOS) was notified that the first brain death investigation was completed and that the heart, lungs, liver, and kidneys were available for donation. All medical institutions nationwide refused to receive the heart because the donor was hepatitis B virus carrier and too old to donate heart; therefore, KONOS selected recipients for lungs, liver and kidneys.
According to a recommendation from the neurologist, electroencephalography (EEG) was performed five times at intervals of 2–3 days before the second brain death examination; however, all EEG findings were not “flat.” Therefore, the brain-death determination process could not progress. However, the family had a strong willingness to donate the patients' organs. Accordingly, DCD was explained after the discontinuation of life-sustaining medical care, and the family agreed to this.
The authors informed KONOS, the Korea Organ and Tissue Donation Agency (KODA), and transplant medical institutions (lung, liver, and two kidneys) about the progress of DCD after WLST. Only the hospital receiving the lung decided to withdraw on learning of DCD; the other selected medical institutions (for the liver and both kidneys) continued with the process. The families agreed that WLST would be performed in the operating room (OR). Before transfer to the OR, however, the family was afforded time to mourn the patient in the ICU.
At 19:30 on July 3, 2020, the patient was transferred to the OR for DCD, and a surgical drape was prepared. The transplant staff waited in surgical gowns in a separate space in a room next to the door with a connecting OR.
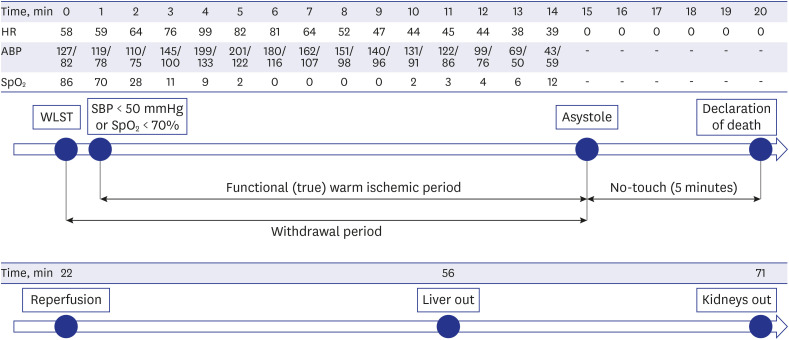
At 20:00, a specialist in charge of critically ill patients, who was also the main physician in charge of the patient, discontinued all life-sustaining treatments, including ventilation, extubated the endotracheal tube, and stopped administration of blood pressure-raising drugs. At 20:01, oxygen saturation (SpO2) fell to < 70%, functional warm ischemic time started, and heart rate arrest (asystole) was confirmed at 20:15. Death was declared by a specialist dedicated to critically ill patients at 20:20, after 5 minutes of “no-touch time” (i.e., from 20:15 to 20:20).
Immediately after death was declared, the medical team of the transplant medical institution, who had been waiting in a separate room, moved to the donor's OR and commenced organ procurement surgery. At 20:22, aortic ligation and histidine-tryptophan-ketoglutarate perfusion were initiated. The liver was removed at 20:56 and both kidneys at 21:11, for donation to the three designated recipients.
Anesthesia and pain medicine medical staff were not present in the OR, and the entire process from the donor entry to the OR to the death of the donor was led by a specialist in charge of critically ill patients. The functional warm ischemia time, defined as the time from systolic blood pressure < 50 mmHg or SpO2 < 70% to asystole, was 15 minutes (20:00 to 20:15), and the total warm ischemic time from the start of WLST to the start of reperfusion was 22 minutes (20:00 to 20:22) (Fig. 1).
Because the present case started as a DBD process, strictly speaking, it fell into DCD category IV. DCD IV is a similar procedure to DCD III if it follows the process of WLST because brain death cannot be determined during the DBD process. If unexpected cardiac arrest is experienced during brain-dead donor management, the procedure is similar to that of DCD V.
In our case, we managed the patient as a brain-dead organ donor. In Korea, the selection of recipients for each organ starts after the completion of the first brain death examination. Therefore, the selection of recipients for the donated organs (lung, liver, and kidneys) was also completed. Because Article 13 of the Enforcement Decree of the “Organic Transplantation Act” restricts organs that can be extracted before the selection of transplant recipients to the eyes, kidneys, pancreas, and islets of brain-dead and deceased persons, it is very important to select transplant recipients before transplantation. In this case, because recipient selection was performed after the first brain death examination, all processes were legal under current domestic law. Therefore, the KONOS allowed organ donation after WLST for the first time in Korea.
There are many cases in which the donation process had begun because the donation of brain-dead organs had been agreed on; however, the actual donation could not be completed in Korea. In South Korea, there were 2,345 brain-dead organ donors in the five years from 2012 to 2016, while 127 patients failed to donate even after consenting to donate brain-dead organs and completion of the primary brain death examination. Reasons included donation incompatibility, cardiac arrest, no brain death, no beneficiaries, refusal to donate, accidental deaths needed legal autopsy, and unknown cause of brain death. For these reasons, potential brain-dead donors died without undergoing the donation operation, and among them, 68 (53.1%) patients could not donate because of death due to cardiac arrest and not brain death. Korea accepted the concept of “whole brain death,” and we have very strict qualifications for brain death determination, such as a “flat” EEG for 30 minutes (< 2 µV).
As such, for patients in whom it was previously not possible to determine brain death or in whom this could not lead to donation due to incomplete brain death determination, DCD category III must be activated. This will not only offer choices to patients and caregivers who decide to donate, but could not due to “no brain death,” even if the patients experience irreversible and unrecoverable brain injury, it will also be another good way for potential recipients who are waiting for a transplant and on the brink of death not to die due to an imbalance between organ supply and demand.
In the future, even if they are no potential brain deaths, patient groups who agree to WLST should be actively asked to donate and, if there is a willingness to do so, it should be possible to implement DCD category III in a true sense. For that purpose, even if the donation process is initiated by consenting to organ donation of the deceased, it is necessary to improve the law, such as by selecting a beneficiary by the National Organ and Tissue Blood Management Service or by expanding the number of organs that can be harvested before selecting a recipient for transplantation. As a result, if the number of organ donations from deceased individuals increases, it is expected that a solution to the imbalance between demand for organ donation and transplantation and death will be provided.
In addition, to revitalize DCD, efforts must be made not only to improve the law but also to reach consensus in government and society. Article 22, Paragraph 3 of the Act on Organs states that organs of brain-dead and deceased persons can be harvested if the person has previously consented or, if the person's intention is not confirmed, with the consent of a legal guardian. To increase the number, only patients who have registered for donation should be deemed to have consented; patients who have not registered for donation should be taken for organ donation only if their legal guardian agrees.
DCD donors can be considered to be individuals who died after death was declared, and no special procedures or processes are required for the declaration of death and organ procurement. However, the criteria for confirming cardiac arrest must be clear, and standard guidelines for DCD need to be established. Although the existing standard of cardiac death is used, there is no clear evidence for observing cardiac arrest for certain period, and if the time is longer, the warm ischemia time that occurs after death is prolonged; as such, donation is practically impossible.
The DCD Research Group has been established since 2019 to revitalize DCD in Korea. The DCD Research Group reviewed the regulations related to DCD in many countries and discussed the ways to revitalize it in Korea, gathering controversial issues, and inquiring about the opinions of the members of the Research Group. All 20 of the 20 medical staff who responded to opinion inquiry at the DCD Research Group agreed to set the no-touch time to 5 minutes in Korea. Based on this, KONOS, KODA, and Korea University Anam Hospital's Organ Transplant Center agreed to set the no-touch time to 5 minutes when DCD was performed after the first life-sustaining treatment in Korea.
No-touch time is the minimal time required to confirm irreversible cessation of circulatory and respiratory functions in DCD. United States DCD protocols use no-touch time of 2–5 minutes.8 The Pittsburgh protocol recommends a no-touch time of 2 minutes. European protocols vary in no-touch times from 5 to 30 minutes.9 According to this survey, 13 out of 18 European countries implementing DCD adopted 5 minutes of no-touch time. Likewise, the no-touch time does not coincide globally.
The United States recommendation is based on the inference that autoresuscitation after 65 seconds of circulatory arrest has never been reported.10 Sheth et al.11 reported retrospectively on 73 donors in controlled NHBD. Donors had a Glasgow coma score of 3 after severe neurological injuries. Circulatory arrest was defined by arterial pulselessness on invasive arterial catheters, absent heart sounds, apnea and unresponsiveness to pain. None recovered arterial pulse at 1 minute. The authors concluded that the 2-minute no-touch time excluded possible autoresuscitation. In 2001, the American College of Critical Care Medicine recommended a no-touch time of 2 to 5 minutes.12
We set the no-touch time by referring to the guidelines of countries around the world. However, since the no-touch time was not constant in all countries, legal and ethical discussions in Korea are needed in the future. In this way, by working with the medical community to improve the law and reach a consensus between government and society, accepting the will of the people and families who wish to donate, the number of organ donations from deceased individuals can be increased.
We expect active implementation of DCD in Korea, resulting in saved lives in those waiting for transplant and decrease the imbalance between organ transplantation and donation.
References
1. Manara AR, Murphy PG, O'Callaghan G. Donation after circulatory death. Br J Anaesth. 2012; 108(Suppl 1):i108–i121. PMID: 22194426.

2. Truog RD, Miller FG, Halpern SD. The dead-donor rule and the future of organ donation. N Engl J Med. 2013; 369(14):1287–1289. PMID: 24088088.

3. Lomero M, Gardiner D, Coll E, Haase-Kromwijk B, Procaccio F, Immer F, et al. Donation after circulatory death today: an updated overview of the European landscape. Transpl Int. 2020; 33(1):76–88. PMID: 31482628.

4. International registry in organ donation and transplantation (IRODaT). Newsletter 2019. Updated 2019. Accessed March 4, 2021. https://www.irodat.org/img/database/pdf/NEWSLETTER2019-June.pdf.
5. Korean Network for Organ Sharing (KONOS). KONOS waiting list in quarterly statistics data. Updated 2019. Accessed March 4, 2021. https://www.konos.go.kr.
6. Kim JM, Kim SJ. The use of non-heart beating donors to expand the donor pool. J Korean Soc Transplant. 2010; 24(3):165–172.

7. National Life Insurance Management Agency. One year of life-sustaining treatment decision system implementation, progress and status of operation. Updated 2019. Accessed March 4, 2021. https://www.lst.go.kr/comm/referenceDetail.do?pgNo=1&cate=&searchOption=0&searchText=&bno=946.
8. Fugate JE, Stadtler M, Rabinstein AA, Wijdicks EF. Variability in donation after cardiac death protocols: a national survey. Transplantation. 2011; 91(4):386–389. PMID: 21127460.

9. Lomero M, Gardiner D, Coll E, Haase-Kromwijk B, Procaccio F, Immer F, et al. Donation after circulatory death today: an updated overview of the European landscape. Transpl Int. 2020; 33(1):76–88. PMID: 31482628.

10. DeVita MA. The death watch: certifying death using cardiac criteria. Prog Transplant. 2001; 11(1):58–66. PMID: 11357558.

11. Sheth KN, Nutter T, Stein DM, Scalea TM, Bernat JL. Autoresuscitation after asystole in patients being considered for organ donation. Crit Care Med. 2012; 40(1):158–161. PMID: 21926577.

12. Ethics Committee, American College of Critical Care Medicine. Society of Critical Care Medicine. Recommendations for nonheartbeating organ donation. A position paper by the Ethics Committee, American College of Critical Care Medicine, Society of Critical Care Medicine. Crit Care Med. 2001; 29(9):1826–1831. PMID: 11546995.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download