INTRODUCTION
Adrenal insufficiency, a clinical syndrome caused by primary adrenal failure or secondary adrenal disease, results in deficient glucocorticoid production or action.
123 It occurs frequently in adults aged ≥ 50 years with the main causes of exogenous corticosteroid use, autoimmune adrenalitis, and tuberculosis.
12 Adrenal insufficiency typically presents as the gradual onset of non-specific symptoms that may include fatigue, loss of appetite, weight loss, nausea and vomiting, abdominal pain, hypotension, and fever.
123 Such symptoms are aggravated in the presence of stressors such as infection and surgical procedures.
23
Because persistent fever often occurs in adrenal insufficiency, it might be confused with infectious diseases in the real-world setting. As a result, patients with adrenal insufficiency and fever might not receive proper treatment timely, leading to prolonged hospitalization as well as the unnecessary use of antibiotics.
Despite relevant studies, little is known about adrenal insufficiency with fever. To ensure timely diagnosis and management, it is necessary to investigate the characteristics of affected patients. This study aimed to identify the clinical characteristics and risk factors of patients with adrenal insufficiency and fever.
Go to :

METHODS
Study setting
This single-center retrospective study was conducted in an 846-bed tertiary hospital in South Korea. All medical records of hospitalized adult patients (age ≥ 19 years) who underwent a standard high-dose adrenocorticotropic hormone (ACTH) stimulation test between 1 March 2018, and 30 June 2019, were reviewed; among them, those who were diagnosed with adrenal insufficiency were recruited. Only the first event per patient was included, and patients were excluded if they had: 1) proven structural problems in the adrenal or pituitary gland; 2) a history of chemotherapy within 6 months prior to the diagnosis of adrenal insufficiency; and 3) other medical conditions that may cause fever, including i) the existence of documented inflammatory disorder, ii) a history of surgery within 4 days prior to the diagnosis,
45 and iii) the existence of concurrent uncontrolled infectious diseases judged by an infectious disease specialist.
Definitions
We used the standard high-dose ACTH stimulation test to diagnose adrenal insufficiency. Serum cortisol was measured at the time of intravenous injection of 250 μg of cosyntropin and repeated at 30 and 60-min post-injection.
6 Adrenal insufficiency was defined as a 30 or 60-min post-injection cortisol level of < 20 μg/dL, while fever was defined as a tympanic temperature of ≥ 37.8°C.
Data collection
We collected data on: 1) demographic features; 2) risk factor variables; 3) initial clinical symptoms; 4) laboratory findings; 5) treatments; and 6) clinical outcomes.
Risk factor variables were derived from the patients' underlying co-morbidities and medical histories. Underlying co-morbidities included components of the Charlson Comorbidity Index
7: myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular accident, dementia, chronic pulmonary disease, connective tissue disease, peptic ulcer disease, liver disease, diabetes mellitus, hemiplegia, chronic kidney disease, solid tumor, leukemia, lymphoma, and acquired immunodeficiency syndrome. Additional medical history items included renal replacement therapy, a history of infectious disease within 1 month, a history of surgery within 6 months, prior use of corticosteroids, use of steroids, use of antibiotics, and use of immunosuppressants.
Initial clinical symptoms at the time of diagnosis included general weakness, confusion, headache, dizziness, skin rash, edema, myalgia, gastrointestinal symptoms (abdominal pain, nausea, vomiting, diarrhea, constipation, hepatosplenomegaly), cardiovascular symptoms (chest pain), and pulmonary symptoms (cough, dyspnea).
The laboratory data at the time of diagnosis were collected for basal and 30 and 60-min post-infusion cortisol, ACTH, thyroid stimulating hormone, free T4, C-reactive protein (CRP), sodium, potassium, blood urea nitrogen, creatinine, albumin, calcium, and glucose levels and white blood cell and eosinophil counts.
For treatment, information about low-dose steroid (potency equivalent to prednisolone < 15 mg) or high-dose steroid (potency equivalent to prednisolone ≥ 15 mg), a change of antibiotics, use of nonsteroidal anti-inflammatory drugs (NSAIDs), and use of immunosuppressants was collected.
To assess outcomes, we evaluated days to defeverence, CRP level (at 3 and 7 days after the diagnosis of adrenal insufficiency), all-cause mortality at 30 days after admission, and hospitalization period from the diagnosis of adrenal insufficiency.
Statistical analysis
All statistical analyses were performed using SPSS version 21.0 for Windows (IBM Corporation, Armonk, NY, USA). Categorical variables were analyzed by the χ2 test or Fisher's exact test. Continuous variables were analyzed using the Mann–Whitney U-test or independent t-test. A multivariable logistic regression analysis was performed to evaluate the effect of the independent variables. Variables with P values < 0.1 on univariate analysis were examined. To avoid collinearity among the variables, we adopted a stepwise regression method with backward elimination. Using a two-tailed test, P values < 0.05 were considered statistically significant.
Ethics statement
The study protocol was approved by the Institutional Review Board (IRB) of Hanyang University Hospital (IRB No. 2019-05-036-001), in compliance with Declaration of Helsinki, The Belmont Report, CIOMS Guidelines and The International Practice (ICH-GCP). All methods were performed in accordance with these guidelines and regulations. The requirement for written informed consent from patients was waived due to the study's retrospective nature.
Go to :

RESULTS
A total of 959 adult inpatients who underwent standard high-dose ACTH stimulation testing were screened; of them, 41.9% (402/959) were diagnosed with adrenal insufficiency. Among them, 252 cases were excluded: 41 with repeated events per patient, 16 with proven structural problems in the adrenal or pituitary gland, 12 with a history of chemotherapy within 6 months prior to the diagnosis of adrenal insufficiency, and 183 with other medical conditions that may cause fever. Finally, 150 cases were included: 45 (30.0%) with fever and 105 (70.0%) without fever at the time of diagnosis of adrenal insufficiency (
Fig. 1).
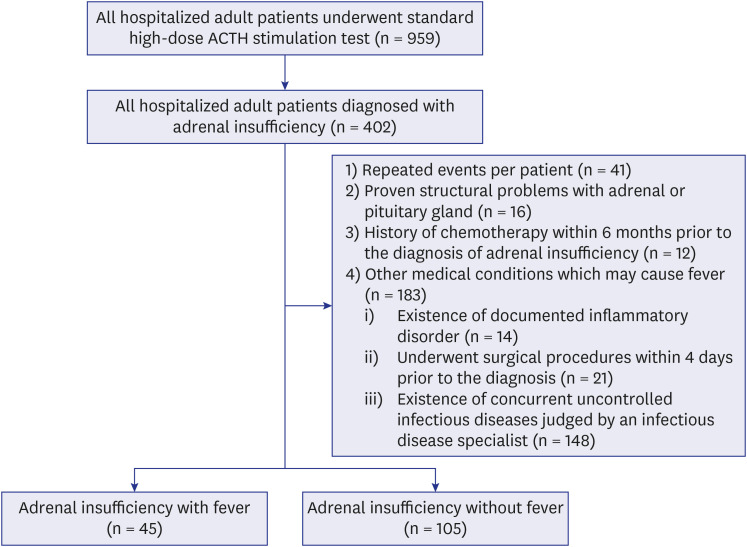 | Fig. 1
Flow diagram showing the patient selection process.
ACTH = adrenocorticotropic hormone.

|
Clinical characteristics
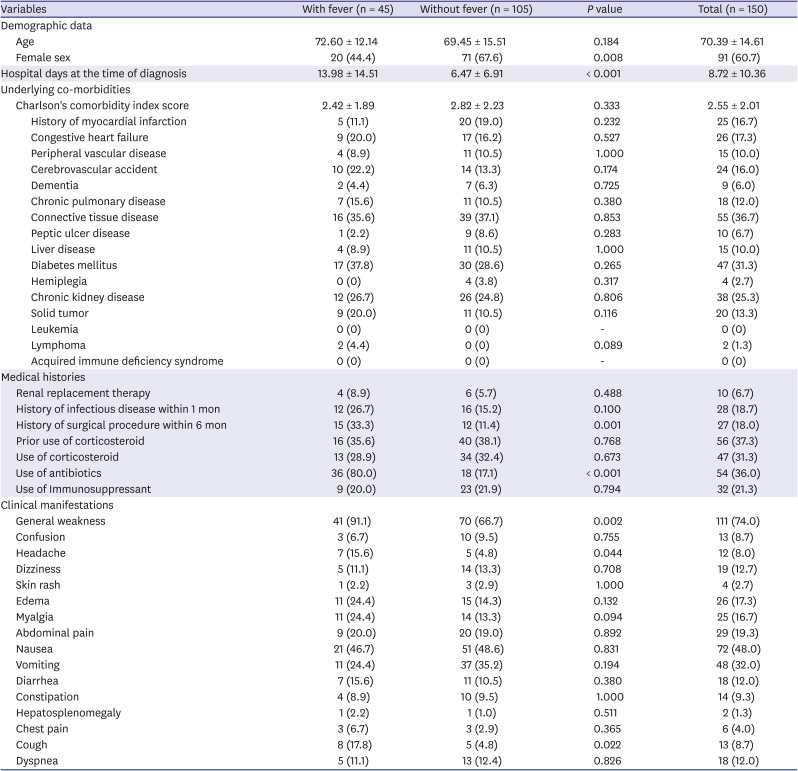
The overall clinical characteristics of the study participants are shown in
Table 1. The ratio of adrenal insufficiency patients with fever to those without fever was 3:7. The mean patient age was 70.39 ± 14.61 years; 60.7% (91/150) of them were women. The mean hospital day at the time of diagnosis was 8.72 ± 10.36 days, while the mean Charlson Comorbidity Index score was 2.55 ± 2.01. The proportion of patients who used corticosteroids and antibiotics was 31.3% and 36.0%, respectively. The most common clinical manifestation was general weakness (74.0%), followed by nausea (38.0%) and vomiting (32.0%).
Table 1
Comparison of clinical characteristics of adrenal insufficiency patients combined with fever to those without fever
|
Variables |
With fever (n = 45) |
Without fever (n = 105) |
P value |
Total (n = 150) |
|
Demographic data |
|
|
|
|
|
Age |
72.60 ± 12.14 |
69.45 ± 15.51 |
0.184 |
70.39 ± 14.61 |
|
Female sex |
20 (44.4) |
71 (67.6) |
0.008 |
91 (60.7) |
|
Hospital days at the time of diagnosis |
13.98 ± 14.51 |
6.47 ± 6.91 |
< 0.001 |
8.72 ± 10.36 |
|
Underlying co-morbidities |
|
|
|
|
|
Charlson's comorbidity index score |
2.42 ± 1.89 |
2.82 ± 2.23 |
0.333 |
2.55 ± 2.01 |
|
|
History of myocardial infarction |
5 (11.1) |
20 (19.0) |
0.232 |
25 (16.7) |
|
|
Congestive heart failure |
9 (20.0) |
17 (16.2) |
0.527 |
26 (17.3) |
|
|
Peripheral vascular disease |
4 (8.9) |
11 (10.5) |
1.000 |
15 (10.0) |
|
|
Cerebrovascular accident |
10 (22.2) |
14 (13.3) |
0.174 |
24 (16.0) |
|
|
Dementia |
2 (4.4) |
7 (6.3) |
0.725 |
9 (6.0) |
|
|
Chronic pulmonary disease |
7 (15.6) |
11 (10.5) |
0.380 |
18 (12.0) |
|
|
Connective tissue disease |
16 (35.6) |
39 (37.1) |
0.853 |
55 (36.7) |
|
|
Peptic ulcer disease |
1 (2.2) |
9 (8.6) |
0.283 |
10 (6.7) |
|
|
Liver disease |
4 (8.9) |
11 (10.5) |
1.000 |
15 (10.0) |
|
|
Diabetes mellitus |
17 (37.8) |
30 (28.6) |
0.265 |
47 (31.3) |
|
|
Hemiplegia |
0 (0) |
4 (3.8) |
0.317 |
4 (2.7) |
|
|
Chronic kidney disease |
12 (26.7) |
26 (24.8) |
0.806 |
38 (25.3) |
|
|
Solid tumor |
9 (20.0) |
11 (10.5) |
0.116 |
20 (13.3) |
|
|
Leukemia |
0 (0) |
0 (0) |
- |
0 (0) |
|
|
Lymphoma |
2 (4.4) |
0 (0) |
0.089 |
2 (1.3) |
|
|
Acquired immune deficiency syndrome |
0 (0) |
0 (0) |
- |
0 (0) |
|
Medical histories |
|
|
|
|
|
Renal replacement therapy |
4 (8.9) |
6 (5.7) |
0.488 |
10 (6.7) |
|
History of infectious disease within 1 mon |
12 (26.7) |
16 (15.2) |
0.100 |
28 (18.7) |
|
History of surgical procedure within 6 mon |
15 (33.3) |
12 (11.4) |
0.001 |
27 (18.0) |
|
Prior use of corticosteroid |
16 (35.6) |
40 (38.1) |
0.768 |
56 (37.3) |
|
Use of corticosteroid |
13 (28.9) |
34 (32.4) |
0.673 |
47 (31.3) |
|
Use of antibiotics |
36 (80.0) |
18 (17.1) |
< 0.001 |
54 (36.0) |
|
Use of Immunosuppressant |
9 (20.0) |
23 (21.9) |
0.794 |
32 (21.3) |
|
Clinical manifestations |
|
|
|
|
|
General weakness |
41 (91.1) |
70 (66.7) |
0.002 |
111 (74.0) |
|
Confusion |
3 (6.7) |
10 (9.5) |
0.755 |
13 (8.7) |
|
Headache |
7 (15.6) |
5 (4.8) |
0.044 |
12 (8.0) |
|
Dizziness |
5 (11.1) |
14 (13.3) |
0.708 |
19 (12.7) |
|
Skin rash |
1 (2.2) |
3 (2.9) |
1.000 |
4 (2.7) |
|
Edema |
11 (24.4) |
15 (14.3) |
0.132 |
26 (17.3) |
|
Myalgia |
11 (24.4) |
14 (13.3) |
0.094 |
25 (16.7) |
|
Abdominal pain |
9 (20.0) |
20 (19.0) |
0.892 |
29 (19.3) |
|
Nausea |
21 (46.7) |
51 (48.6) |
0.831 |
72 (48.0) |
|
Vomiting |
11 (24.4) |
37 (35.2) |
0.194 |
48 (32.0) |
|
Diarrhea |
7 (15.6) |
11 (10.5) |
0.380 |
18 (12.0) |
|
Constipation |
4 (8.9) |
10 (9.5) |
1.000 |
14 (9.3) |
|
Hepatosplenomegaly |
1 (2.2) |
1 (1.0) |
0.511 |
2 (1.3) |
|
Chest pain |
3 (6.7) |
3 (2.9) |
0.365 |
6 (4.0) |
|
Cough |
8 (17.8) |
5 (4.8) |
0.022 |
13 (8.7) |
|
Dyspnea |
5 (11.1) |
13 (12.4) |
0.826 |
18 (12.0) |

Although there was no difference in the proportion of underlying co-morbidities including the Charlson Comorbidity Index score, there were significant differences in several clinical characteristics between patients with and without fever. The proportion of female patients was significantly lower among patients with fever than among those without fever (44.4% vs. 67.6%, P = 0.008). In addition, patients with fever were diagnosed at 13.98 ± 14.51 days, later than those without fever (6.47 ± 6.91 days) (P < 0.001). More patients with fever had a history of surgery within 6 months (33.3% vs. 11.4%, P = 0.001) and used antibiotics at the time of diagnosis (80.0% vs. 17.1%, P < 0.001) than those without fever. Among the clinical manifestations, general weakness (91.1% vs. 66.7%, P = 0.002), headache (15.6% vs. 4.8%, P = 0.044), and cough (17.8% vs. 4.8%, P = 0.022) were more frequently observed in patients with fever than in those without fever.
Initial laboratory findings
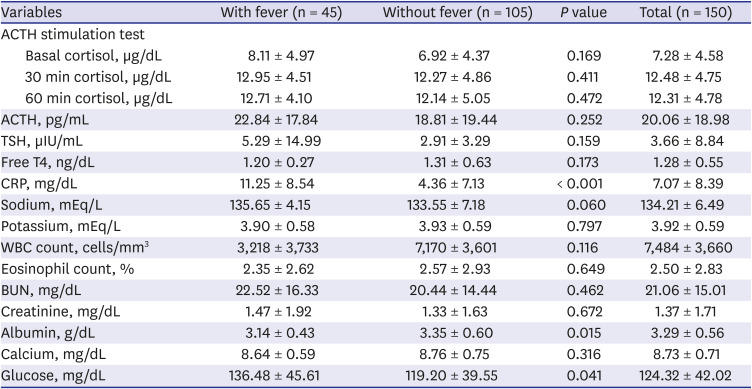
The overall initial laboratory findings are shown in
Table 2. There were no significant intergroup differences in random basal or 30 and 90-min cortisol levels after standard high-dose ACTH stimulation tests. In comparison, the mean CRP level was higher in patients with fever than in those without fever (11.25 ± 8.54 vs. 4.36 ± 7.13 mg/dL,
P < 0.001). In addition, a lower serum albumin level (3.14 ± 0.43 vs. 3.35 ± 0.60 g/dL,
P = 0.015) and higher serum glucose level (136.48 ± 45.61 vs. 119.20 ± 39.55 mg/dL,
P = 0.041) were observed in patients with fever than in those without fever.
Table 2
Comparison of initial laboratory findings characteristics of adrenal insufficiency patients combined with fever to those without fever
|
Variables |
With fever (n = 45) |
Without fever (n = 105) |
P value |
Total (n = 150) |
|
ACTH stimulation test |
|
|
|
|
|
Basal cortisol, µg/dL |
8.11 ± 4.97 |
6.92 ± 4.37 |
0.169 |
7.28 ± 4.58 |
|
30 min cortisol, µg/dL |
12.95 ± 4.51 |
12.27 ± 4.86 |
0.411 |
12.48 ± 4.75 |
|
60 min cortisol, µg/dL |
12.71 ± 4.10 |
12.14 ± 5.05 |
0.472 |
12.31 ± 4.78 |
|
ACTH, pg/mL |
22.84 ± 17.84 |
18.81 ± 19.44 |
0.252 |
20.06 ± 18.98 |
|
TSH, µIU/mL |
5.29 ± 14.99 |
2.91 ± 3.29 |
0.159 |
3.66 ± 8.84 |
|
Free T4, ng/dL |
1.20 ± 0.27 |
1.31 ± 0.63 |
0.173 |
1.28 ± 0.55 |
|
CRP, mg/dL |
11.25 ± 8.54 |
4.36 ± 7.13 |
< 0.001 |
7.07 ± 8.39 |
|
Sodium, mEq/L |
135.65 ± 4.15 |
133.55 ± 7.18 |
0.060 |
134.21 ± 6.49 |
|
Potassium, mEq/L |
3.90 ± 0.58 |
3.93 ± 0.59 |
0.797 |
3.92 ± 0.59 |
|
WBC count, cells/mm3
|
3,218 ± 3,733 |
7,170 ± 3,601 |
0.116 |
7,484 ± 3,660 |
|
Eosinophil count, % |
2.35 ± 2.62 |
2.57 ± 2.93 |
0.649 |
2.50 ± 2.83 |
|
BUN, mg/dL |
22.52 ± 16.33 |
20.44 ± 14.44 |
0.462 |
21.06 ± 15.01 |
|
Creatinine, mg/dL |
1.47 ± 1.92 |
1.33 ± 1.63 |
0.672 |
1.37 ± 1.71 |
|
Albumin, g/dL |
3.14 ± 0.43 |
3.35 ± 0.60 |
0.015 |
3.29 ± 0.56 |
|
Calcium, mg/dL |
8.64 ± 0.59 |
8.76 ± 0.75 |
0.316 |
8.73 ± 0.71 |
|
Glucose, mg/dL |
136.48 ± 45.61 |
119.20 ± 39.55 |
0.041 |
124.32 ± 42.02 |

Clinical outcomes
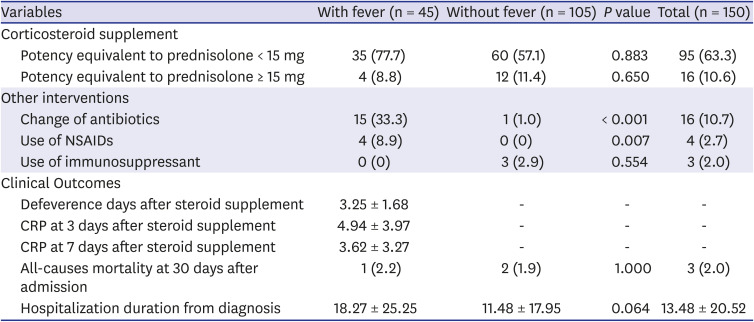
Table 3 shows the treatment and clinical outcomes of the two groups. A total of 111 (74.0%) patients received corticosteroid supplements. Of them, 63.3% (95/150) received the equivalent of prednisolone < 15 mg, while 10.6% (16/150) received the equivalent of prednisolone ≥ 15 mg. There were no significant intergroup differences in corticosteroid supplementation.
Table 3
Comparison of clinical outcomes of adrenal insufficiency patients combined with fever to those without fever
|
Variables |
With fever (n = 45) |
Without fever (n = 105) |
P value |
Total (n = 150) |
|
Corticosteroid supplement |
|
|
|
|
|
Potency equivalent to prednisolone < 15 mg |
35 (77.7) |
60 (57.1) |
0.883 |
95 (63.3) |
|
Potency equivalent to prednisolone ≥ 15 mg |
4 (8.8) |
12 (11.4) |
0.650 |
16 (10.6) |
|
Other interventions |
|
|
|
|
|
Change of antibiotics |
15 (33.3) |
1 (1.0) |
< 0.001 |
16 (10.7) |
|
Use of NSAIDs |
4 (8.9) |
0 (0) |
0.007 |
4 (2.7) |
|
Use of immunosuppressant |
0 (0) |
3 (2.9) |
0.554 |
3 (2.0) |
|
Clinical Outcomes |
|
|
|
|
|
Defeverence days after steroid supplement |
3.25 ± 1.68 |
- |
- |
- |
|
CRP at 3 days after steroid supplement |
4.94 ± 3.97 |
- |
- |
- |
|
CRP at 7 days after steroid supplement |
3.62 ± 3.27 |
- |
- |
- |
|
All-causes mortality at 30 days after admission |
1 (2.2) |
2 (1.9) |
1.000 |
3 (2.0) |
|
Hospitalization duration from diagnosis |
18.27 ± 25.25 |
11.48 ± 17.95 |
0.064 |
13.48 ± 20.52 |

A total of 16 (10.7%) patients changed antibiotics; most had fever (93.7% [15/16]). A higher proportion of patients with fever changed antibiotics (33.3% vs. 1.0%, P < 0.001) and used NSAIDs (8.9% vs. 0%, P = 0.007).
As for clinical outcomes, 2.0% of patients had died by 30 days after admission, and the mean hospitalization duration from the diagnosis of adrenal insufficiency was 13.48 ± 20.52 days. There were no significant intergroup differences in clinical outcomes. Among the patients with fever, the mean time to defeverence after the diagnosis of adrenal insufficiency was 3.25 ± 1.68 days.
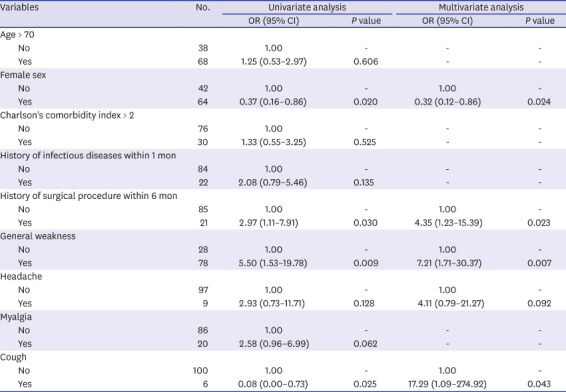
Risk factors for adrenal insufficiency with fever
The relative risks determined by multivariate analysis are shown in
Table 4. Female sex (odds ratio [OR], 0.32; 95% confidence interval [CI], 0.12–0.86;
P = 0.024) lowered the risk of adrenal insufficiency with fever. In comparison, a history of surgery within 6 months (OR, 4.35; 95% CI, 1.23–15.39;
P = 0.023), general weakness (OR, 7.21; 95% CI, 1.71–30.37;
P = 0.007), and cough (OR, 17.29; 95% CI, 1.09–274.92;
P = 0.043) were significantly associated with adrenal insufficiency with fever.
Table 4
Risk factors for adrenal insufficiency with fever using a multivariable logistic regression model

|
Variables |
No. |
Univariate analysis |
Multivariate analysis |
|
OR (95% CI) |
P value |
OR (95% CI) |
P value |
|
Age > 70 |
|
|
|
|
|
|
No |
38 |
1.00 |
- |
- |
- |
|
Yes |
68 |
1.25 (0.53–2.97) |
0.606 |
- |
- |
|
Female sex |
|
|
|
|
|
|
No |
42 |
1.00 |
- |
1.00 |
- |
|
Yes |
64 |
0.37 (0.16–0.86) |
0.020 |
0.32 (0.12–0.86) |
0.024 |
|
Charlson's comorbidity index > 2 |
|
|
|
|
|
|
No |
76 |
1.00 |
- |
- |
- |
|
Yes |
30 |
1.33 (0.55–3.25) |
0.525 |
- |
- |
|
History of infectious diseases within 1 mon |
|
|
|
|
|
|
No |
84 |
1.00 |
- |
- |
- |
|
Yes |
22 |
2.08 (0.79–5.46) |
0.135 |
- |
- |
|
History of surgical procedure within 6 mon |
|
|
|
|
|
|
No |
85 |
1.00 |
- |
1.00 |
- |
|
Yes |
21 |
2.97 (1.11–7.91) |
0.030 |
4.35 (1.23–15.39) |
0.023 |
|
General weakness |
|
|
|
|
|
|
No |
28 |
1.00 |
- |
1.00 |
- |
|
Yes |
78 |
5.50 (1.53–19.78) |
0.009 |
7.21 (1.71–30.37) |
0.007 |
|
Headache |
|
|
|
|
|
|
No |
97 |
1.00 |
- |
1.00 |
- |
|
Yes |
9 |
2.93 (0.73–11.71) |
0.128 |
4.11 (0.79–21.27) |
0.092 |
|
Myalgia |
|
|
|
|
|
|
No |
86 |
1.00 |
- |
- |
- |
|
Yes |
20 |
2.58 (0.96–6.99) |
0.062 |
- |
- |
|
Cough |
|
|
|
|
|
|
No |
100 |
1.00 |
- |
1.00 |
- |
|
Yes |
6 |
0.08 (0.00–0.73) |
0.025 |
17.29 (1.09–274.92) |
0.043 |

Go to :

DISCUSSION
To the best of our knowledge, this is the first study to describe the clinical characteristics of adrenal insufficiency with fever. In this study, 30% of patients with adrenal insufficiency had a fever, and the mean CRP level was significantly higher in patients with fever than in those without fever. Fever and CRP level elevation are associated with inflammatory cytokine production.
8 When fever-producing substances stimulate granulocytes and macrophages of the reticuloendothelial system, a heat-labile peptide called endogenous pyrogen is produced, resulting in the initiation of a cascade of processes via phospholipase A
2 activation and consequent production of prostaglandin E
2, which finally produces the set-point displacement of body temperature when the prostaglandin E
2 injected into the hypothalamus.
8 CRP is synthesized by hepatocytes after acute inflammatory stimuli and is principally regulated at the transcriptional level by the cytokines interleukin-6 and interleukin-1β.
9 Adrenal insufficiency might activate these processes; however, the underlying mechanisms are yet to be determined.
Of the patients with fever, approximately 65% had not used corticosteroids before. Given that a large proportion of adrenal insufficiency is caused by the withdrawal of long-term corticosteroid therapy, the possibility of inaccurate past history existed. Some patients might not have been aware of taking long-term corticosteroids and having adrenal insufficiency. In Korea, before the implementation of the ‘separation of drug prescribing and dispensing’ policy in 2000, inappropriate prescription of systemic corticosteroids without proper notification to patients was prevalent.
10 The possibility of primary adrenal insufficiency should be considered as well. Autoimmune adrenalitis is the most common cause of primary adrenal insufficiency (80–90% of cases in developed countries).
12 Autoimmune adrenalitis may accompany fever, increased CRP levels, and decreased serum albumin levels as a result of microdestruction of the adrenal gland.
11121314 Further studies are necessary to clarify these issues.
In this study, patients with fever were diagnosed with adrenal insufficiency at mean 7.5 days later than those without fever. The most plausible explanation for this phenomenon is that most physicians suspect infectious disease first when they encounter a febrile patient. Therefore, the diagnosis of adrenal insufficiency might be delayed, and physicians might change the antibiotic class with the aim of treating an infectious disease without addressing the differential diagnosis. In fact, the antibiotics were changed in more than 30% of the adrenal insufficiency patients with fever in the present study. Such inappropriate antibiotic use not only causes drug side effects in patients, but also increases medical costs and contributes to the emergence of antimicrobial-resistant bacteria.
15 Physicians should be aware of the potential risk of antibiotic misuse considering the fact that the effectiveness of antibiotics reduces once a pathogen acquires resistance, leading to increased mortality rates.
16 Because of the increase in antimicrobial-resistant pathogens, more than 35,000 people die annually in the US.
17 Therefore, thorough efforts to investigate the differential diagnoses of adrenal insufficiency are required, especially when patients with a fever of unknown origin have risk factors such as male sex, a history of surgery within 6 months, general weakness, and cough.
When a steroid was administered to the adrenal insufficiency patients with fever in this study, defeverence was achieved in a mean of 3.25 days. Although it is unclear whether steroid supplementation affects long-term outcomes, it might help physicians rule out the possibility of an infectious disease in an adrenal insufficiency patient with fever. Further studies of the effect of steroid supplements on these patients are necessary.
Our study had some limitations. First, the possibility of adrenal insufficiency was not evaluated for patients who did not undergo ACTH stimulation testing so that the prevalence of fever in adrenal insufficiency might have been overestimated. Second, because of the nature of retrospective studies, some patients' symptoms might be not recorded properly in their medical records. Also, there might be subjective differences in the physicians' judgments about their patients' symptoms. Furthermore, there is a possibility that patients with infectious diseases or inflammatory disorders might have not been fully excluded due to the nature of the retrospective study. To minimize the possibility of the inclusion of those patients, all the medical records were reviewed and confirmed by an infectious disease specialist. Third, long-term follow-up of the patients' clinical features was not performed and the effects of the interventions such as steroid supplementation were not adequately evaluated. Thus, further studies on the long-term clinical features and effectiveness of the interventions are needed. Fourth, this study was performed in a tertiary-care hospital and the data of only hospitalized patients were collected. Therefore, our results may not be generalizable to other settings. Despite these limitations, the overall data of this study are likely to be a reasonable approximation of the true values; thus, our study can aid in the precise diagnosis of adrenal insufficiency in febrile patients, providing cost-effective benefits for patients and reducing unnecessary antibiotic use.
In conclusion, the possibility of adrenal insufficiency should be considered in patients with fever of unknown origin and risk factors such as male sex, a history of surgery within 6 months, general weakness, and cough.
Go to :









 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download