INTRODUCTION
The coronavirus disease 2019 (COVID-19) epidemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), first occurred in Wuhan, China, in December 2019, and was declared a pandemic by the World Health Organization (WHO) in March 2020.
1 Globally, 100 million people have been infected with SARS-CoV-2, and healthcare workers (HCWs) are particularly at risk of infection with SARS-COV-2.
2 For protection against the virus, appropriate personal protective equipment (PPE) is essential, not optional. Masks or respirators provide respiratory protection and are important in protecting HCWs from airborne and droplet infectious diseases. Due to the possibility of an airborne transmission of SARS-CoV-2, masks or respirators are crucial, especially during aerosol-producing procedures such as tracheal suction and intubation.
345
Respirator leakage and fitting are issues that should not be overlooked for appropriate level of protection, other than the type of respirators. The Centers for Disease Control and Prevention (CDC) reported rate of leakage due to the mismatch between the respirator and the HCWs' facial structure from 6–88%, and the protective effect against the infectious agent could be reduced by 33% if not properly adhered.
6 Previous Korean studies that investigated the protective effect of N95 respirators against infectious agents, reported high leakage and low fitting rates.
78 Many factors such as sex, age, shape of the face, breathing flow rate, and wearer's movement affect the extent of respirator leakage.
910111213 Respirators used in healthcare facilities vary in types and shapes, and the HCWs' face shapes also vary. Therefore, it is necessary to select a respirator that fits the HCWs' face shape through the fit test, and check whether there is leakage before the HCWs' exposure to a patient with the source of infection.
In Korea, most N95 respirators are imported, especially from the United States of America (USA); therefore, domestic N95 respirators are an important alternative to maintain adequate infection management during this prolonged pandemic. In addition, in Korea, the standard medical masks are classified as Korean Filter (KF), and the KF94 mask is judged to have an effect similar to or better than that of the N95 respirator.
714 According to the Korean government PPE recommendations, the KF94 mask is at the same level as the N95 respirator and a KF94 mask should be worn when contacting a patient with COVID-19.
15 To overcome the shortage of N95 respirators, the only choice is to utilize the domestic N95 respirators and KF94 masks in the medical field.
16 However, no previous research exists on the leakage and fitting rates of the KF94 mask, and only one study was performed on domestic N95 respirators.
17
In this study, we performed the fit tests for made in Korea KF94 masks and N95 respirators among HCWs and evaluated whether the masks were adequately fitted and protective. In addition, for N95 respirators, adequate fitting rates, were compared between those made in Korea and those made in the USA.
Go to :

METHODS
Study design and study population
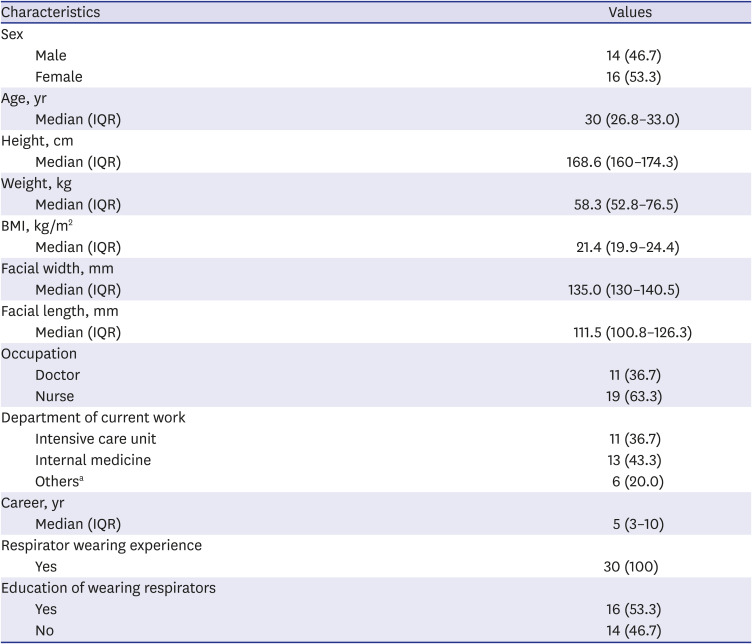
This prospective single-center simulation study included HCWs who provided care for COVID-19 patients or worked in departments with high exposure risk to COVID-19 patients at Hallym University Kangnam Sacred Heart Hospital. A total of 30 volunteers over 19 years of age, regardless of sex, occupation, position, or career, were recruited for two months from December 1, 2020 to January 31, 2021.
Participants' characteristics
All participants were surveyed through a questionnaire for age, sex, occupation, current work department, career, experience with wearing masks or respirators, previous education on how to properly wear respirators, height, and weight. The body mass index (BMI) was calculated from the height and weight. Facial width (bizygomatic breadth) and length (menton-sellion length) were measured.
Equipment and materials
Five N95 respirators and six KF94 masks were tested. N95 respirators were classified as made in Korea or the USA. Among fourteen domestic National Institute for Occupational Safety and Health (NIOSH)-approved N95 respirators, those by two domestic companies, three N95 respirators representing each company without valve available for purchase by domestic delivery, and two N95 respirators made by 3M (a representative respirator production company in the USA), were examined. Regarding the domestic N95 respirators, we acquired C250 (Ever Green, Uiwang, Korea), 201 (DOBU LIFE TECH, Gwangju, Korea), and 500 (DOBU LIFE TECH, Gwangju, Korea). Regarding the 3M respirators, we acquired one commonly used model (1860; 3M, St. Paul, MN, USA) and one-fold type model (9210+; 3M, St. Paul, MN, USA). Among the KF94 masks in circulation, six randomly selected KF94 masks by the researcher were divided into either horizontal (n = 3) or vertical (n = 3) folding types, and two large and one medium-sized masks were examined.
A two-fit test device was used. The quantitative fit test for the respirator and medical mask was performed using the PortaCount Plus 8048 device (TSI Inc., Shoreview, MN, USA). This device simultaneously measures the concentration of particles in ambient air and the concentration of particles inside the respirator. The fit factor (FF) is calculated from the ratio of the concentration in the respirator or mask to the concentration in the ambient air. FF scores ranged from 1 to 200. An FF > 200 was shown as 200 by the device. An FF ≥ 100 was defined as adequate protection. The MT-03 (Sibata Scientific Technology Ltd., Japan) device measured the particle count in the respirator or mask and ambient air and calculated the leakage rate. The leakage rate (%) of particles into the mask is obtained by calculating the particles in the mask or respirator divided by ambient particles, multiplied by 100. A leakage rate of ≤ 5 was defined as adequate protection. This value was calculated by assuming that the ratio of the inhalation and exhalation times were 1:1.2, based on the standard leakage rate of ≤ 11% in the KF94 mask's license standard, by the Korean Food and Drug Administration.
14
Intervention
All the masks and respirators for all the participants were tested using both devices (PortaCount Plus 8048 and MT-03). Smoking, eating, and drinking were prohibited for ≥ 30 minutes before the test. If the participants had a beard, that was shaved. The test was performed in an independent room in an intensive care unit (ICU) without an operating air-conditioning system.
The tests were performed during the following four exercises, according to the new protocol of the Occupational Safety and Health Administration: 1) bending over for 50 seconds, 2) talking for 30 seconds, 3) turning the head from side to side for 30 seconds, and 4) moving the head up and down for 39 seconds; for each device. For the KF94 mask, each process was repeated after fixing the ear strap with a hook at the back of the head.
18
Each test had a washout period of approximately 10 minutes to reduce errors due to physical loss.
Study outcomes and data analysis
The primary outcome was adequate protection rate of N95 respirators and KF94 masks. Adequate protection rate was calculated for each equipment as the percentage of overall FF ≥ 100 using the PortaCount Plus 8048 device and an overall leakage rate of ≤ 5 by MT-03. The overall FF was calculated as follows:
Where FFn is the fit factor of n movement of one participant.
In addition, the overall leakage rate was calculated as follows:
Where N is the n movement of one participant.
The secondary outcome was the comparison of adequate protection rates of N95 respirators manufactured in Korea and by 3M in the USA.
Statistical analysis
The participants' general characteristics and body information were analyzed using descriptive statistics. Categorical data are reported as absolute numbers and percentages, and continuous data are reported as median values and interquartile ranges (IQRs). Statistical significance was assessed using the χ2 test, McNemar's test, or Fisher's exact test for categorical variables. Continuous variables were analyzed using the Mann-Whitney U test and Wilcoxon signed rank test. Spearman correlation analysis was performed to analyze the associations between the FF and leakage rate. Logistic regression analysis was performed to identify factors contributing to adequate protection. Univariate and multivariate analyses were performed using stepwise backward selection procedures. Statistical significance was set at P < 0.05. Statistical analysis was performed using the IBM Statistical Package for the Social Sciences Statistics for Windows, version 20 (IBM Corp., Armonk, NY, USA).
Ethics statement
The study was approved by the Institutional Review Board of Hallym University Kangnam Sacred Heart Hospital in Korea (2020-06-011-006), and informed consent was obtained from all participants.
Go to :

DISCUSSION
In Korea, medical masks are marked using the KF standard. KF94 masks are considered to have similar efficacy as N95 respirators, and KF94 masks have been recommended for HCWs in the era of emerging infectious diseases, especially the COVID-19 pandemic.
71415 To protect HCWs from infectious diseases, the importance of fitting the respirators and medical masks has been reported; however, the fit test for the KF94 mask has not been conducted.
19 In this study, we performed a fit test and evaluated the adequate protection rates of N95 respirators and KF94 masks. To our knowledge, this is the first study performed on the fit test for the KF94 masks, including the domestic N95 respirators made in Korea.
In response to COVID-19, the Korean guidelines recommended the wearing of KF94 masks or N95 respirators in aerosol-generating procedures, such as tracheal suction and intubation.
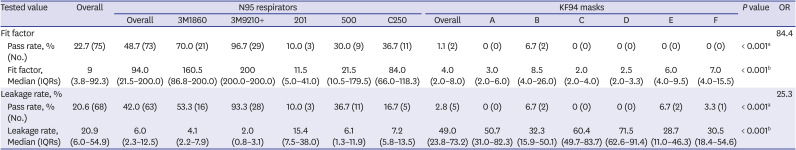
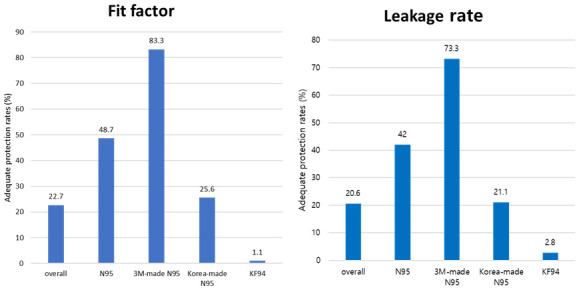
15 If appropriate fitting is required for proper protection with the use of N95 respirators, the same applies for KF94 masks. In this study, when classifying N95 respirators and KF94 masks, the N95 respirators showed a significantly higher adequate protection rate than that of KF94 masks (48.7% vs. 1.1% in FF and 42.0% vs. 2.8% in leakage rate). This implies that even if the filtering efficiency is similar, particles can leak through the worn masks. These results raised a question regarding the stability of KF94 masks during aerosol-generating procedures. Wearing a KF94 mask in an improperly fitted state while performing procedures suggest a risk of exposure to aerosol; thus, it is necessary to verify the fit while wearing the KF94 mask through a more accurate method or Powered Air Purifying Respirator above KF94 should be worn for more optimal protection. Many factors can explain the difference between N95 respirators and KF94 masks; however, the main difference in the structure is the shape of the strap. While the N95 respirators have a head-band design, the KF94 mask used in this study had an ear-loop design. The ear-loop design appears to be less effective for a proper fit compared with the head-band design.
20 The results of this study, with a better fit when a hook is used to fix the loop at the back of the head, could be an evidence of a structural problem. In addition, the part of the mask in direct contact with the face was thin and lifts easily with movement. Most of the KF94 masks could not be tightened with a string, so they could not be adjusted to an individual's face. When HCWs wear the KF94 mask, it is necessary to improve the fit by checking that the masks fit well to the size of the HCWs face in advance; researching the method of wearing a mask to prevent leakage is therefore necessary.
To date, many studies have tested the fit of N95 respirators targeting Korean HCWs, and the results varied.
1721222324 In the most recent study conducted using a Korean N95 respirator, 5.9% showed a good fit, and the average overall FF was 27.3.
17 In previous studies in emergency medical centers, 73.7–100% of proper fit and FF scores of 27 to 93 were reported, depending on the type of respirator.
212223 In a study of nurses and nursing students, 73.5% showed adequate protection.
24 In this study, the adequate protection rate of N95 respirators in the fit test was 48.7%, is lower than that reported in previous studies (73.5–100%).
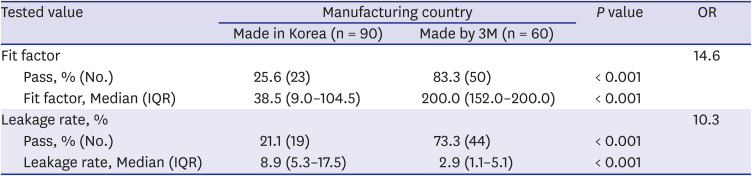
1721222324 However, most previous studies were performed using 3M respirators. The adequate protection rate of 3M respirators of 83.3% and the median FF of 200 in this study, are similar to or higher than those in a previous study. Only one study targeted a domestic N95 respirator.
17 This study included three types of domestic N95 respirators, and the adequate protection rate of 25.6% reported in this study was higher than that in a previous study that included a domestic N95 respirator.
17 From the results of this study, the adequate protection rate was significantly lower than that of 3M N95 respirators. As the COVID-19 pandemic is being prolonged, it is necessary to prepare measures to address the shortage of PPE, and domestic N95 respirators, which are important alternatives. However, there is a risk that HCWs might use N95 respirators while caring for COVID-19 patients, with inadequate fit. Participants had a median career of 5 years, and most of them worked in the ICU and internal medicine sections; therefore, they should have been familiar with N95 respirators' use, especially 3M N95 respirators, which are widely used. Although the participants had experience wearing a domestic N95 respirator (the only one type of respirator was C250), this was the first time they used it in 2020. The experience is insufficient compared with that with the 3M respirators. To properly use the domestic N95 respirators in the actual clinical field, it is important to improve the degree of adequate protection by repeated wearing of domestic N95 respirators. Although 3M respirators showed a more appropriate fit, it was not a perfect fit. This should also be improved.
In one study, previous fit testing was not a predictor of the appropriate fit test results, and among those who repeatedly used it, the fitting rate was high.
25 This suggests that a fit test is useful for finding a mask suitable for individuals but does not guarantee that the fitting will work well afterwards. In clinical practice, to ensure safety, it is important that HCWs are properly fitted every time they are at risk of exposure to an infection source. The existing fit test method by FF may be difficult to use in real-world clinical settings because it involves partial destruction (the creation of a hole) of the respirator, compromising the protection offered by the equipment during contact with a patient. Conversely, the method used for leakage rate does not compromise the structural integrity of the equipment and, therefore, can be performed before patient contact. In this study, a correlation analysis was performed to determine the association between FF and leakage rate. The FF and leakage rate correlated negatively (R = −0.778,
P < 0.001). The leakage rate can be used as a non-destructive method at every entry, by the HCWs. For the safety of the HCWs, it should be considered necessary to periodically check the appropriate respirators by the fit test and check the suitability of the leakage test before they encounter patients with infectious pathogens.
There are many factors that affect the leakage rate of respirators, including gender, face shape, and respirator design.
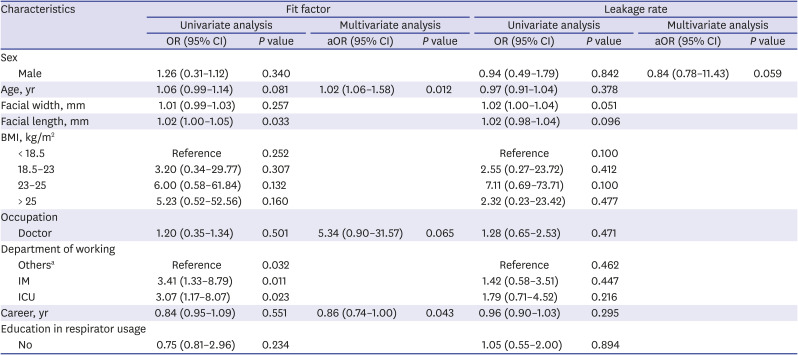
9101126 In this study, only age and career affected adequate protection to FF and no factor affected the leakage rate. The shorter the working career, the higher the adequate protection, which is similar to the results of a previous domestic survey.
27 Although access to education on the wearing of respirators did not affect adequate protection, in some previous reports, education improved proper fit.
2328 It might be that the efforts to understand and do this correctly with the relatively recent training on wearing of respiratory protection equipment had worked. Age was another factor in this study, but the aOR was 1.02; therefore, the effect would be negligible.
Our study had several limitations. First, this study was conducted in a single institution. Therefore, this might not reflect the state of all HCWs in medical institutions in Korea. Second, the types of masks and respirators used in this study were few. N95 respirators were selected as the most used or made in Korea respirators. KF94 masks that could be used in Korea were not only varied in type, but some were also sold out during the study period. We chose the mask type available at the time of the study. Additional testing may be required for various types of masks and respirators. Third, only four actions were performed, which were somewhat different from the techniques performed in actual clinical practice. Although it was performed as an action based on the Occupational Safety and Health Administration for the fit test, it is necessary to investigate the actions to see what changes actually occurred in the real clinical field. Fourth, we did not classify the respirator size according to the facial size. All N95 respirators used in the study were medium-sized, and small respirators were not used. However, in the actual clinical settings, medium-sized respirators are mostly used. Fifth, each test was performed just once for each respirator or mask. As mentioned above, repeated wearing will improve adequate fitting, and more accurate fitting rate can be obtained through repeated tests. Therefore, further repeated tests should be performed.
In conclusion, although the adequate protection rate of N95 respirators was higher than that of KF94 masks, the protection of N95 respirator was not optimum. It is necessary to minimize exposure to risk by selecting an appropriate mask or respirators that adequately fit each person and ensure that respirators or masks are worn properly before contacting patients. Based on our findings, we recommend wearing N95 respirators instead of KF94 masks because of their superior protection rate, especially when performing aerosol-generating procedures.
Go to :






 PDF
PDF Citation
Citation Print
Print



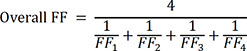
 XML Download
XML Download