INTRODUCTION
Pediatric sedative upper endoscopy (PSUE) requires more caution as the respiratory system of children is more likely to be fragile than those of adults. Although similar equipment is used, the insertion of the endoscopy tube leads to airway obstruction more often in children than in adults. Drugs that increase salivation are more likely to cause respiratory difficulty in children, and a higher dose per bodyweight of sedative drugs is required to be effective compared with that in adults.
1 PSUE requires an appropriate selection of drugs, as well as an appropriate level of sedation, which is consistent with pediatric procedural sedation guidelines and the special circumstances of endoscopy. Moreover, careful monitoring is necessary to enable an immediate intervention for adverse events.
According to previous reviews, sedative drugs used in PSUE include midazolam, propofol, ketamine, and opioids such as fentanyl and meperidine, and they may be used either alone or in combination.
23 In South Korea, PSUE, which involves a method slightly different from that recommended by global pediatric guidelines but offers good endoscopic access similar to that in adult endoscopy, is used in pediatric endoscopy. Deep sedation is important for the success of a procedure, but it also increases the risk of complications, such as respiratory difficulty. As such, safety protocols in the literature propose the use of general anesthesia or deep sedation in the presence of an anesthesiologist.
45 However, most Korean pediatric endoscopists perform procedural sedation without an anesthesiologist. Korean pediatric endoscopists make case-by-case drug and dose selections to find a balance between vital sign stability and the ease of procedures.
Korean pediatric endoscopists can receive training in approximately 10 training centers and are practicing in pediatric hospitals across the country. Due to the differences in the training environment and preferences for sedation methods, the sedation method for PSUE may differ slightly across institutions and continually evolve. Until now, data regarding PSUE have not been collected in Korea. Thus, this study collected and analyzed the PSUE trends in 15 institutions that performed pediatric endoscopy for the past year. This study aimed to review the literature to find an appropriate sedative method for PSUE and analyze the effectiveness and complications of the available methods based on the type, combination, and dose of sedative drugs used. This will be useful to physicians who wish to perform pediatric endoscopy.
Go to :

METHODS
Patients and methods
From June 1, 2019, to May 31, 2020, the medical records of patients undergoing pediatric esophagogastroduodenoscopy (EGD) under sedation at 15 general and tertiary hospitals in South Korea were retrospectively reviewed. One or more pediatric endoscopists were on duty at each hospital (endoscopic unit). The collected data included age, sex, height, weight, body mass index, reasons for endoscopy or final diagnosis, underlying disease, and medication history. The assessment of the EGD procedures also determined whether the duodenum was fully observed and the time of the procedure. We checked whether pediatric endoscopy was performed with or without sedation; if it was performed without sedation, the reasons were analyzed. The combinations and dosages of sedative drugs were investigated for cases of sedation.
Drugs used for sedation, such as midazolam, ketamine, propofol, pethidine, and lidocaine, were investigated, but pethidine and fentanyl were used as analgesics, and they were excluded from the analysis in this study. Based on the combination of drugs used, the patients were allocated to five groups: (a) midazolam, ketamine, and propofol group; (b) midazolam and propofol group; (c) midazolam and ketamine group; (d) propofol alone (continuous infusion for 20 minutes) group; (e) midazolam alone group. The doses of the drugs, degrees of sedation, and complications in each group were assessed.
The degree of sedation was evaluated using the Ramsay sedation scale with 1–6 points. The Ramsey score is a subjective tool that can be used to evaluate the level of consciousness during the titration of sedative medication. The scores are categorized as follows: 1, anxious, agitated, or restless responses; 2, cooperative, oriented, and tranquil responses; 3, response to commands only; 4, brisk response to a light glabellar tap or loud auditory stimulus; 5, sluggish response to a light glabellar tap or loud auditory stimulus; 6, no response to a light glabellar tap or loud auditory stimulus.
6
Complications, such as irritability, hiccups, convulsions, desaturation (by pulse oximetry below 90%), increased oxygen supply during the procedure, changes in blood pressure, bag-mask ventilation, intubation, and cardiopulmonary resuscitation (CPR), during sedation were analyzed. The factors affecting the degree of sedation were also investigated.
Statistical analysis
Data were considered statistically significant if their P values were below 0.05. All statistical analyses were performed using R software (version 4.0.2). For statistical comparison of the groups, the Student's t-test or Mann-Whitney U test was used for continuous variables, and the χ2 test or Fisher's exact test was used for categorical variables. Comparative data for continuous variables are reported as mean and standard deviation. Univariable logistic regression analysis was first conducted to investigate the crude odds ratio (OR) for each factor. A multivariate logistic regression analysis was conducted using the variables with P values below 0.05 that were used in the univariable logistic regression. The results were expressed as ORs with 95% confidence intervals (CIs).
Ethics statement
This study was approved by the Institutional Review Boards (IRBs) of all the participating centers (Jeonbuk National University Hospital IRB No.2020-06-023), and informed consent was waived because of the retrospective review of the medical records.
Go to :

RESULTS
Seven hundred and ninety-nine cases of pediatric EGD were performed alone at 15 university medical hospitals. Seven hundred and thirty-four patients who underwent conscious sedation (procedural sedation) were included in the study, except 58 cases of endoscopy without sedation, 5 with general anesthesia, 1 with failed endoscopy, and 1 with missing Ramsey scale scores (
Fig. 1). Fifty-eight of 799 (7.3%) cases of EGDs were performed without sedation for children younger than 2 years (30 of 799, 3.8%) or those with unstable vital signs (16 of 799, 2.0%). Fifteen patients (1.9%) underwent endoscopic foreign body removal without sedation.
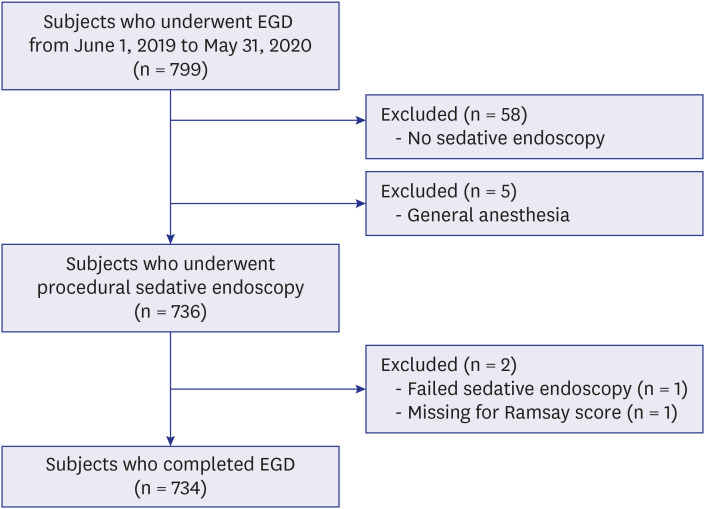 | Fig. 1
Flow chart of subject selection.
EGD = esophagogastroduodenoscopy.

|
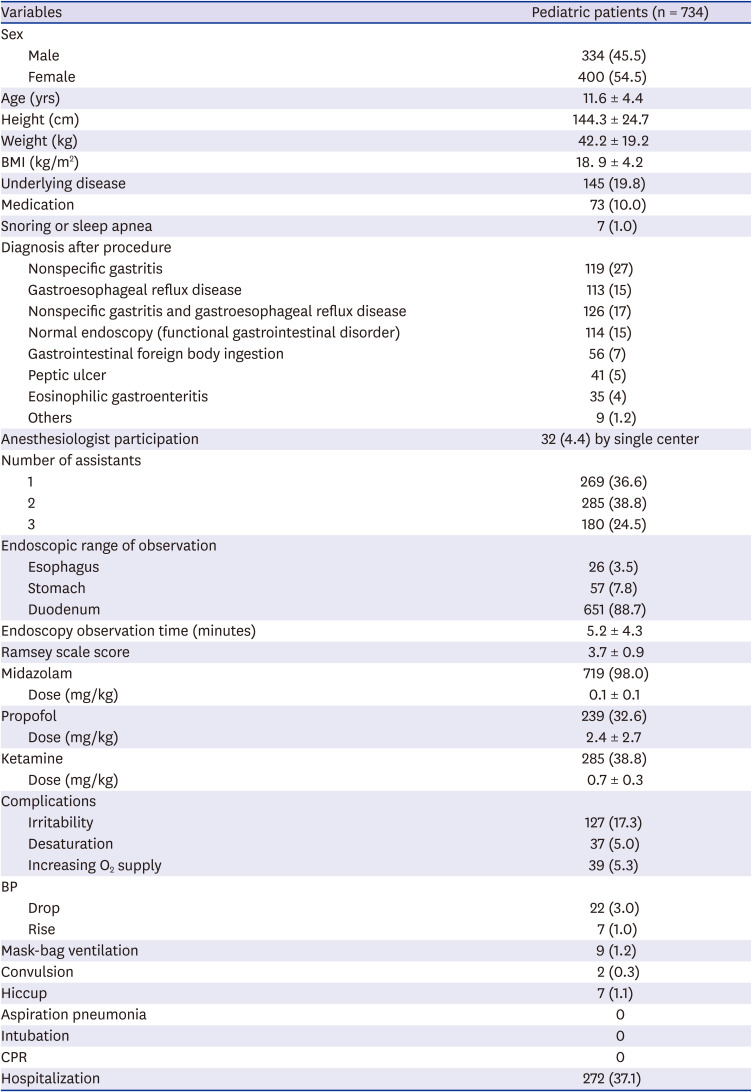
The median age group ranged from two weeks to 19 years (average 11.6 years old); 400 cases were female and 334 were male. One hundred and forty-five patients (19.8%) had underlying disease, 73 patients (10%) had been taking medications such as anticonvulsant drugs, and 7 cases (1%) snored or had sleep apnea. The final diagnoses of patients who underwent EGD with sleep sedation in this study were as follows: gastritis, 199 (27%); and gastroesophageal reflux disease, 113 (15%). Of the patients, 126 (17%) had gastroesophageal reflux disease and gastritis, 114 (15%) had symptoms but their endoscopic findings were normal, 56 (7%) had gastrointestinal foreign body ingestion, 41 (5%) had gastric ulcers, 35 (4%) had eosinophilic gastritis, and 9 (1.2%) had inflammatory bowel disease. Other diagnoses (9 patients, 1.2%) included esophageal varix, Mallory-Weiss syndrome, esophageal candidiasis, and submucosal tumor (
Table 1).
Table 1
Baseline characteristics of patients undergoing esophagogastroduodenoscopy under sedation
|
Variables |
Pediatric patients (n = 734) |
|
Sex |
|
|
Male |
334 (45.5) |
|
Female |
400 (54.5) |
|
Age (yrs) |
11.6 ± 4.4 |
|
Height (cm) |
144.3 ± 24.7 |
|
Weight (kg) |
42.2 ± 19.2 |
|
BMI (kg/m2) |
18. 9 ± 4.2 |
|
Underlying disease |
145 (19.8) |
|
Medication |
73 (10.0) |
|
Snoring or sleep apnea |
7 (1.0) |
|
Diagnosis after procedure |
|
|
Nonspecific gastritis |
119 (27) |
|
Gastroesophageal reflux disease |
113 (15) |
|
Nonspecific gastritis and gastroesophageal reflux disease |
126 (17) |
|
Normal endoscopy (functional gastrointestinal disorder) |
114 (15) |
|
Gastrointestinal foreign body ingestion |
56 (7) |
|
Peptic ulcer |
41 (5) |
|
Eosinophilic gastroenteritis |
35 (4) |
|
Others |
9 (1.2) |
|
Anesthesiologist participation |
32 (4.4) by single center |
|
Number of assistants |
|
|
1 |
269 (36.6) |
|
2 |
285 (38.8) |
|
3 |
180 (24.5) |
|
Endoscopic range of observation |
|
|
Esophagus |
26 (3.5) |
|
Stomach |
57 (7.8) |
|
Duodenum |
651 (88.7) |
|
Endoscopy observation time (minutes) |
5.2 ± 4.3 |
|
Ramsey scale score |
3.7 ± 0.9 |
|
Midazolam |
719 (98.0) |
|
Dose (mg/kg) |
0.1 ± 0.1 |
|
Propofol |
239 (32.6) |
|
Dose (mg/kg) |
2.4 ± 2.7 |
|
Ketamine |
285 (38.8) |
|
Dose (mg/kg) |
0.7 ± 0.3 |
|
Complications |
|
|
Irritability |
127 (17.3) |
|
Desaturation |
37 (5.0) |
|
Increasing O2 supply |
39 (5.3) |
|
BP |
|
|
Drop |
22 (3.0) |
|
Rise |
7 (1.0) |
|
Mask-bag ventilation |
9 (1.2) |
|
Convulsion |
2 (0.3) |
|
Hiccup |
7 (1.1) |
|
Aspiration pneumonia |
0 |
|
Intubation |
0 |
|
CPR |
0 |
|
Hospitalization |
272 (37.1) |

The mean duration for EGD was 5.2 ± 4.3 seconds. Of the patients, 88.7% (651 patients) were observed up to the duodenum, 7.8% (57 patients) were observed to the stomach, and 3.5% (26 patients) were observed to the esophagus. Sedation was implemented by anesthesiologists in 32 cases (4.4%); the rest were sedated by pediatric endoscopists. The pediatric endoscopic specialists were assisted by nurses or residents; there was one assistant for 36.6% (269 of 734) of the cases, two for 38.8% (285 of 734) of the cases, and three for 24.5% (180 of 734) of the cases. Midazolam was used in 719 cases (98%) at a mean dosage of 0.1 mg/kg. Ketamine was used in 285 cases (38.8%) at a mean dosage of 0.7 mg/kg, and propofol was used in 239 cases (32.6%) at a mean dosage of 2.4 mg/kg. The mean Ramsey score was 4. During EGD with sedation, irritability was observed in 127 patients (17.3%); 37 patients (5.0%) showed decreased oxygen saturation, and 39 patients (5.3%) had an increased oxygen supply during the procedure. Mask-bag ventilation was performed in nine patients (1.2%), and there was no intubation or CPR. Two patients showed convulsions during endoscopy, and seven patients (1.2%) had hiccup symptoms. A total of 272 (37.1%) patients were hospitalized before and after endoscopy; 109 (14.9%) were hospitalized for endoscopy and other examinations, and 158 (21.5%) were hospitalized to control the disease and symptoms. Four (0.5%) patients were hospitalized to control complications (
Table 1).
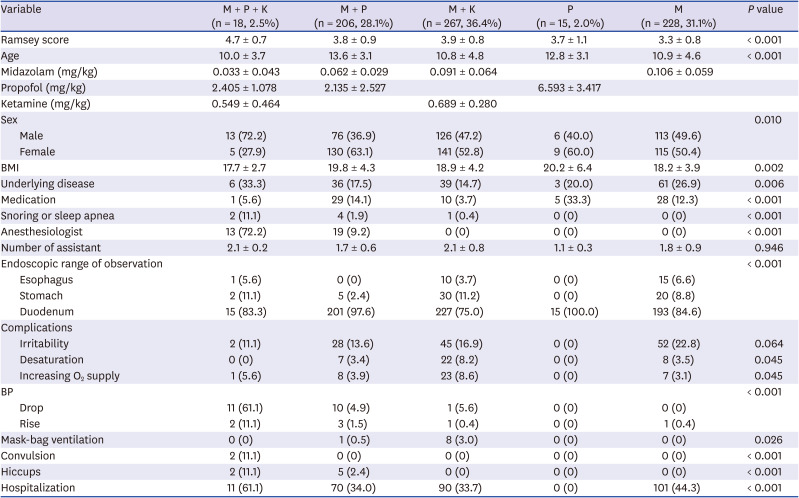
The combination of midazolam and ketamine was the most commonly used (267 cases; 36.4%), and the mean Ramsey scale score was 3.9. In the group using midazolam alone, 228 (31.1%) had a mean Ramsey score of 3.3. The midazolam and propofol group included 206 patients (28.1%), with a mean Ramsay score of 3.8. The midazolam, propofol, and ketamine group included 18 patients (2.5%), with a mean Ramsey scale score of 4.7. There were 15 patients (2.0%) in the group using continuous infusion of propofol alone for approximately 20 minutes, and their mean Ramsey score was 3.7 (
Table 2). The ages of the group using midazolam and propofol (13.6) and the group using propofol alone (12.8) were higher than those of the other groups. Although the number of patients was only 15, endoscopy was used to explore up to the duodenum in all patients in the propofol alone group. In the group using midazolam, propofol, and ketamine, 15 of 18 (83.3%) patients were observed up to the duodenum. A total of 193 of 228 (84.6%) patients in the midazolam group were observed up to the duodenum (
Table 2).
Table 2
Baseline characteristics according to drug combinations
|
Variable |
M + P + K (n = 18, 2.5%) |
M + P (n = 206, 28.1%) |
M + K (n = 267, 36.4%) |
P (n = 15, 2.0%) |
M (n = 228, 31.1%) |
P value |
|
Ramsey score |
4.7 ± 0.7 |
3.8 ± 0.9 |
3.9 ± 0.8 |
3.7 ± 1.1 |
3.3 ± 0.8 |
< 0.001 |
|
Age |
10.0 ± 3.7 |
13.6 ± 3.1 |
10.8 ± 4.8 |
12.8 ± 3.1 |
10.9 ± 4.6 |
< 0.001 |
|
Midazolam (mg/kg) |
0.033 ± 0.043 |
0.062 ± 0.029 |
0.091 ± 0.064 |
|
0.106 ± 0.059 |
|
|
Propofol (mg/kg) |
2.405 ± 1.078 |
2.135 ± 2.527 |
|
6.593 ± 3.417 |
|
|
|
Ketamine (mg/kg) |
0.549 ± 0.464 |
|
0.689 ± 0.280 |
|
|
|
|
Sex |
|
|
|
|
|
0.010 |
|
Male |
13 (72.2) |
76 (36.9) |
126 (47.2) |
6 (40.0) |
113 (49.6) |
|
Female |
5 (27.9) |
130 (63.1) |
141 (52.8) |
9 (60.0) |
115 (50.4) |
|
BMI |
17.7 ± 2.7 |
19.8 ± 4.3 |
18.9 ± 4.2 |
20.2 ± 6.4 |
18.2 ± 3.9 |
0.002 |
|
Underlying disease |
6 (33.3) |
36 (17.5) |
39 (14.7) |
3 (20.0) |
61 (26.9) |
0.006 |
|
Medication |
1 (5.6) |
29 (14.1) |
10 (3.7) |
5 (33.3) |
28 (12.3) |
< 0.001 |
|
Snoring or sleep apnea |
2 (11.1) |
4 (1.9) |
1 (0.4) |
0 (0) |
0 (0) |
< 0.001 |
|
Anesthesiologist |
13 (72.2) |
19 (9.2) |
0 (0) |
0 (0) |
0 (0) |
< 0.001 |
|
Number of assistant |
2.1 ± 0.2 |
1.7 ± 0.6 |
2.1 ± 0.8 |
1.1 ± 0.3 |
1.8 ± 0.9 |
0.946 |
|
Endoscopic range of observation |
|
|
|
|
|
< 0.001 |
|
Esophagus |
1 (5.6) |
0 (0) |
10 (3.7) |
0 (0) |
15 (6.6) |
|
Stomach |
2 (11.1) |
5 (2.4) |
30 (11.2) |
0 (0) |
20 (8.8) |
|
Duodenum |
15 (83.3) |
201 (97.6) |
227 (75.0) |
15 (100.0) |
193 (84.6) |
|
Complications |
|
|
|
|
|
|
|
Irritability |
2 (11.1) |
28 (13.6) |
45 (16.9) |
0 (0) |
52 (22.8) |
0.064 |
|
Desaturation |
0 (0) |
7 (3.4) |
22 (8.2) |
0 (0) |
8 (3.5) |
0.045 |
|
Increasing O2 supply |
1 (5.6) |
8 (3.9) |
23 (8.6) |
0 (0) |
7 (3.1) |
0.045 |
|
BP |
|
|
|
|
|
< 0.001 |
|
Drop |
11 (61.1) |
10 (4.9) |
1 (5.6) |
0 (0) |
0 (0) |
|
Rise |
2 (11.1) |
3 (1.5) |
1 (0.4) |
0 (0) |
1 (0.4) |
|
Mask-bag ventilation |
0 (0) |
1 (0.5) |
8 (3.0) |
0 (0) |
0 (0) |
0.026 |
|
Convulsion |
2 (11.1) |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
< 0.001 |
|
Hiccups |
2 (11.1) |
5 (2.4) |
0 (0) |
0 (0) |
0 (0) |
< 0.001 |
|
Hospitalization |
11 (61.1) |
70 (34.0) |
90 (33.7) |
0 (0) |
101 (44.3) |
< 0.001 |

Irritability was most frequently observed in the group using midazolam alone (52 patients, 22.8%). Hiccups were observed in seven patients; two were in the midazolam, propofol, and ketamine group, and 5 were in the group using midazolam and propofol. Convulsions occurred in two patients who used midazolam, propofol, and ketamine (
Table 2).
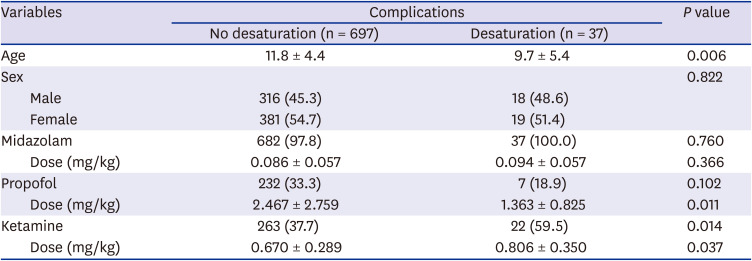
Desaturation below 90% was the highest in 22 cases (8.2%) in the midazolam and ketamine group (
P = 0.045), and the highest oxygen supply was observed in 23 patients (8.6%) in the midazolam and ketamine group (
P = 0.045). Blood pressure decreased in 11 of 15 (61.1%) patients in the midazolam, propofol, and ketamine group. Blood pressure increased in 2 cases (11.1%) in the midazolam, propofol, and ketamine group and 3 cases (1.5%) in the midazolam and propofol group (
P < 0.001). Mask-bag ventilation was most commonly used in the midazolam and ketamine group (8 patients, 3%,
P = 0.026,
Table 2).
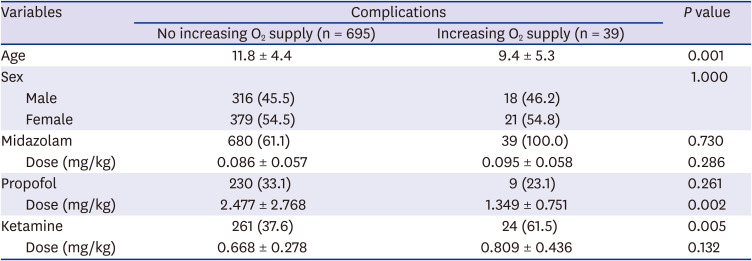
Six hundred of 734 (81.7%) patients underwent endoscopy with sedation and concomitant oxygen administration. Thirty-nine patients (5%) had an increased oxygen supply during sedation. The increase in oxygen supply was significantly higher when ketamine was used (
P = 0.005) and in younger patients (
P = 0.001). The use of propofol was not significantly related to the increase in oxygen supply (
P = 0.261), but the dose of propofol was related (
P = 0.002). The use and dosage of midazolam were not significantly related to the increase in oxygen supply (
Tables 3,
4,
5,
6). Desaturation was observed in 37 patients (5.0%), and it also significantly increased when ketamine was used (
P = 0.014). There was a correlation between the dose of ketamine (
P = 0.037) and younger age (
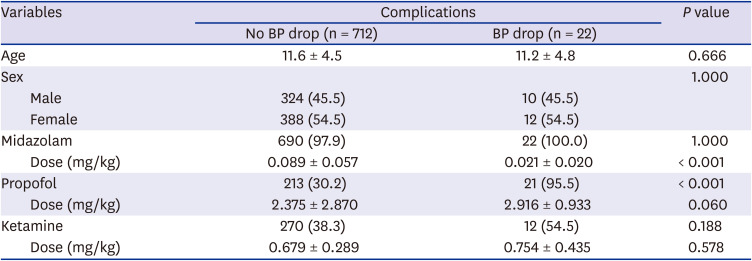
P = 0.006). There was a correlation between the decrease in blood pressure and the use of propofol (
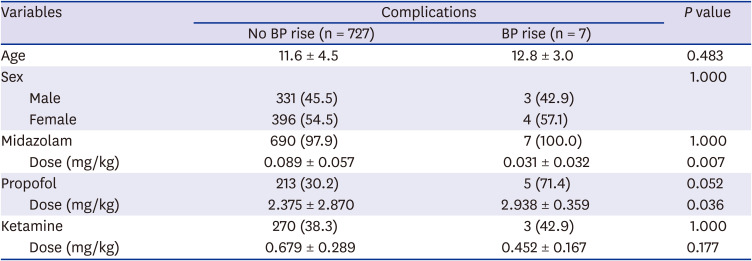
P < 0.001). Blood pressure was elevated in seven patients (1.0%), and it was correlated with the dose of propofol (
P = 0.036;
Tables 3-
6).
Table 3
The sedatives and doses of drugs following complications of pediatric upper endoscopy
|
Variables |
Complications |
P value |
|
No increasing O2 supply (n = 695) |
Increasing O2 supply (n = 39) |
|
Age |
11.8 ± 4.4 |
9.4 ± 5.3 |
0.001 |
|
Sex |
|
|
1.000 |
|
Male |
316 (45.5) |
18 (46.2) |
|
Female |
379 (54.5) |
21 (54.8) |
|
Midazolam |
680 (61.1) |
39 (100.0) |
0.730 |
|
Dose (mg/kg) |
0.086 ± 0.057 |
0.095 ± 0.058 |
0.286 |
|
Propofol |
230 (33.1) |
9 (23.1) |
0.261 |
|
Dose (mg/kg) |
2.477 ± 2.768 |
1.349 ± 0.751 |
0.002 |
|
Ketamine |
261 (37.6) |
24 (61.5) |
0.005 |
|
Dose (mg/kg) |
0.668 ± 0.278 |
0.809 ± 0.436 |
0.132 |

Table 4
The sedatives and doses of drugs following complications of pediatric upper endoscopy
|
Variables |
Complications |
P value |
|
No desaturation (n = 697) |
Desaturation (n = 37) |
|
Age |
11.8 ± 4.4 |
9.7 ± 5.4 |
0.006 |
|
Sex |
|
|
0.822 |
|
Male |
316 (45.3) |
18 (48.6) |
|
Female |
381 (54.7) |
19 (51.4) |
|
Midazolam |
682 (97.8) |
37 (100.0) |
0.760 |
|
Dose (mg/kg) |
0.086 ± 0.057 |
0.094 ± 0.057 |
0.366 |
|
Propofol |
232 (33.3) |
7 (18.9) |
0.102 |
|
Dose (mg/kg) |
2.467 ± 2.759 |
1.363 ± 0.825 |
0.011 |
|
Ketamine |
263 (37.7) |
22 (59.5) |
0.014 |
|
Dose (mg/kg) |
0.670 ± 0.289 |
0.806 ± 0.350 |
0.037 |

Table 5
The sedatives and doses of drugs following complications of pediatric upper endoscopy
|
Variables |
Complications |
P value |
|
No BP drop (n = 712) |
BP drop (n = 22) |
|
Age |
11.6 ± 4.5 |
11.2 ± 4.8 |
0.666 |
|
Sex |
|
|
1.000 |
|
Male |
324 (45.5) |
10 (45.5) |
|
Female |
388 (54.5) |
12 (54.5) |
|
Midazolam |
690 (97.9) |
22 (100.0) |
1.000 |
|
Dose (mg/kg) |
0.089 ± 0.057 |
0.021 ± 0.020 |
< 0.001 |
|
Propofol |
213 (30.2) |
21 (95.5) |
< 0.001 |
|
Dose (mg/kg) |
2.375 ± 2.870 |
2.916 ± 0.933 |
0.060 |
|
Ketamine |
270 (38.3) |
12 (54.5) |
0.188 |
|
Dose (mg/kg) |
0.679 ± 0.289 |
0.754 ± 0.435 |
0.578 |

Table 6
The sedatives and doses of drugs following complications of pediatric upper endoscopy
|
Variables |
Complications |
P value |
|
No BP rise (n = 727) |
BP rise (n = 7) |
|
Age |
11.6 ± 4.5 |
12.8 ± 3.0 |
0.483 |
|
Sex |
|
|
1.000 |
|
Male |
331 (45.5) |
3 (42.9) |
|
Female |
396 (54.5) |
4 (57.1) |
|
Midazolam |
690 (97.9) |
7 (100.0) |
1.000 |
|
Dose (mg/kg) |
0.089 ± 0.057 |
0.031 ± 0.032 |
0.007 |
|
Propofol |
213 (30.2) |
5 (71.4) |
0.052 |
|
Dose (mg/kg) |
2.375 ± 2.870 |
2.938 ± 0.359 |
0.036 |
|
Ketamine |
270 (38.3) |
3 (42.9) |
1.000 |
|
Dose (mg/kg) |
0.679 ± 0.289 |
0.452 ± 0.167 |
0.177 |

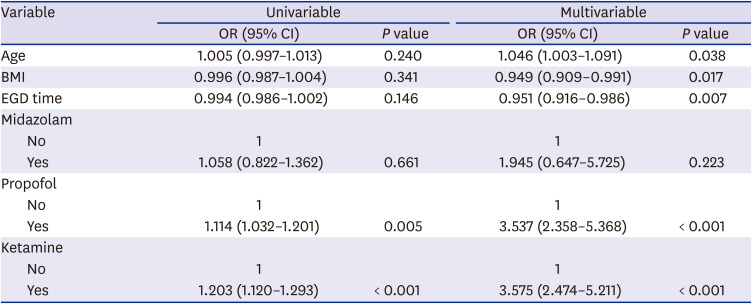
The factors affecting the Ramsey score were significantly related to the use of ketamine, propofol. The degree of sedation was 3.58 times better when ketamine was used than when it was not (
P < 0.001) and 3.54 times better when propofol was used than when it was not (
P < 0.001,
Table 7).
Table 7
Logistic regression analysis for Ramsey sedation scale
|
Variable |
Univariable |
Multivariable |
|
OR (95% CI) |
P value |
OR (95% CI) |
P value |
|
Age |
1.005 (0.997–1.013) |
0.240 |
1.046 (1.003–1.091) |
0.038 |
|
BMI |
0.996 (0.987–1.004) |
0.341 |
0.949 (0.909–0.991) |
0.017 |
|
EGD time |
0.994 (0.986–1.002) |
0.146 |
0.951 (0.916–0.986) |
0.007 |
|
Midazolam |
|
|
|
|
|
No |
1 |
|
1 |
|
|
Yes |
1.058 (0.822–1.362) |
0.661 |
1.945 (0.647–5.725) |
0.223 |
|
Propofol |
|
|
|
|
|
No |
1 |
|
1 |
|
|
Yes |
1.114 (1.032–1.201) |
0.005 |
3.537 (2.358–5.368) |
< 0.001 |
|
Ketamine |
|
|
|
|
|
No |
1 |
|
1 |
|
|
Yes |
1.203 (1.120–1.293) |
< 0.001 |
3.575 (2.474–5.211) |
< 0.001 |

Go to :

DISCUSSION
In this study, the most frequently observed Ramsay scores were 4 (39.0%), 3 (38.8%), and 5 (15.5%) points, demonstrating how sedation during PSUE was performed at a level in which patients were responsive to strong simulation. Irritability was reported in 127 cases (17.3%), which may be attributed to the restraint by assistants during the attempts by the endoscopist to avoid adverse events regardless of the failed procedures. Based on the results of this study, only one case of procedural sedation was performed by an anesthesiologist, which was evident from the Ramsay score indicative of a deeper sedation than that provided on average. In that particular institution, deep sedation was induced under the supervision of an anesthesiologist. The institution had concluded that the recent sedation with midazolam 0.03 mg/kg + ketamine 0.65 mg/kg + propofol 2.5 mg/kg performed by an anesthesiologist was deeper and safer with fewer complications than the previous approaches (midazolam 0.1 mg/kg + ketamine 0.5 mg/kg) performed by pediatric endoscopists.
7 Sedation performed by anesthesiologists is considered desirable by several people. However, due to the cost of pediatric endoscopy and the typical pediatric treatment environment in South Korea, it may be difficult to perform PSUE under such conditions in many institutions. Globally, there have been studies describing sedation with sevoflurane and propofol (1–2 mg/kg bolus or 6–10 mg/kg/h continuous infusion) performed by anesthesiologists who were suitable for pediatric endoscopy.
891011 However, Liu et al.
12 cautiously recommended ketamine + dexmedetomidine and recommended against the use of premedication, inhalational agents, or opioids in children based on the increased risk of upper airway obstruction and concluded that there is limited evidence on sedation provided by an anesthesiologist during pediatric endoscopy. They suggested that propofol may not be the best choice.
12 Based on findings in adults, Riesco-López et al.
13 compared sedative endoscopies with propofol performed by an endoscopist and anesthesiologist, respectively, and concluded that the procedure performed by an endoscopist was more cost-effective for low to intermediate anesthetic risk patients. In a survey of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition members, approximately half responded that sedation for PSUE was performed by an anesthesiologist globally.
4
Regarding the adverse events observed during the procedures in this study, 37 cases (5.0%) of desaturation were reported, and 39 cases (5.3%) required additional oxygen. Although 1–2 L/min of oxygen was provided in most hospitals as a basic protocol for sedative endoscopy (600 cases, 81.7%), 134 cases did not involve a basic supply of oxygen (18.3%). A basic supply of oxygen is deemed necessary for sedative procedures, although a high-flow oxygen supply (over 2 L/kg/min) has not been reported to be more effective for preventing complications than the standard low-flow oxygen supply.
1415 Biber et al.
16 analyzed 12,000 pediatric patients who underwent sedative endoscopy and found no reported cases of cardiac arrest. The reported frequencies of respiratory adverse events (3.3%) were airway obstruction (1.0%), apnea (0.2%), desaturation (1.5%), and laryngospasm (0.6%). Aspiration (0.1%), convulsion (0.1%), non-completion of procedure due to problems (0.2%), secretion (0.4%), change in blood pressure (0.7%), bag-mask ventilation (1.2%), and intubation (0.1%) were also reported.
16 In South Korea, 62 PSUE patients were analyzed, although only reports of airway obstruction/laryngospasm (3.2%) and desaturation (11.3%) were found.
17 The analysis of 30,000 children who underwent pediatric sedative procedures (including upper endoscopy) in the US, Canada, Europe, and Australia between 2004 and 2005 showed the following complications: cardiac arrest (0.003%), convulsion (0.027%), laryngospasm (0.043%), apnea (0.243%), secretions (0.416%), desaturation (1.57%), bag-mask ventilation (0.639%), and intubation (0.097%).
18 Compared with data collected in this study, it is evident that there are more respiratory complications in Korean PSUE than in the other countries. Nonetheless, an absolute comparison may be difficult, as some studies had strict criteria, which may have resulted in the exclusion of some respiratory complications.
Midazolam alone was the second most commonly used (228 cases, 31.1%). Midazolam is an opioid drug belonging to the class of benzodiazepines. It has no analgesic effects, but it is characteristically long-lasting, as its effects are gradual. As such, gradual-onset hypotension may occur, resulting in respiratory depression, and a negative inotropic effect may occur in patients with reduced cardiac function, and this necessitates caution. Paradoxical irritability can also sometimes occur, which may interfere with the procedure. However, the drug has a long-lasting effect (approximately 30 minutes) and an amnesic effect, which can help eliminate negative memories after the procedure.
3 Rafeey et al.
19 reported in a randomized control trial (RCT) that there was no difference between the effects of oral midazolam and intravenous (IV) midazolam during PSUE. Endoscopists who use midazolam alone often perform endoscopy with difficulty and require the help of an assistant to restrain the patient; however, this reflects the strong principle of minimizing adverse effects, including respiratory failure, caused by combining other drugs.
Midazolam + ketamine was identified as the most frequently used drug combination (267 cases, 36.4%). Ketamine has both sedative and analgesic effects, and it is a dissociative anesthetic that inhibits cerebral function and has an amnesic effect. Although patients open their eyes and talk in some cases, they are considered unconscious. The drugs act rapidly, and the duration of their effects is slightly longer than that of propofol at 10 minutes. Midazolam + ketamine has the disadvantages of excess oral secretions, vomiting, and increased intracranial pressure, while its advantages include low rates of respiratory depression and fewer individual differences related to the response to the drug. Furthermore, it may induce pleasant dreams, which may be considered another advantage.
3 The combination of ketamine with midazolam and propofol is recommended to complement the disadvantages of ketamine. The use of midazolam + ketamine is, especially, known for its beneficial drug interactions in the PSUE context.
20 Tosun et al.
21 demonstrated in a small-scale RCT study involving children that propofol + ketamine induced deeper sedation than propofol + fentanyl. However, more adverse events, such as coughing, vomiting, dizziness, and diplopia, have been reported in those sedated with propofol + ketamine.
21 Although the effectiveness of ketamine in inducing deep sedation has been well-demonstrated, caution is needed as it has been significantly associated with the incidence of adverse events, such as desaturation requiring oxygen supplementation, especially in younger patients.
Propofol was used alone for continuous infusion for approximately 20 minutes in 15 cases (2.0%), starting before the procedure until the end of the procedure. As this protocol uses a single drug, a certain dose has to be administered for it to be effective. Propofol is associated with remarkable individual differences in its effective dose with bolus infusions; therefore, continuous infusions allow the examination of patient responses and the individualization of the delivered doses. Nonetheless, it has disadvantages, such as the risk of administering a dose beyond the point at which adverse effects occur and its cumbersome use for a procedure that does not require a long time to complete. Propofol acts very quickly, and its duration of effect is short at approximately 5 minutes. It is not available in patients who are allergic to eggs or soy, and it does not have an analgesic effect similar to that of midazolam.
3 As demonstrated in this study, propofol may induce changes in blood pressure, and caution should be exercised when administering it to patients with poor cardiac function. Vascular pain at the site of IV injection is a disadvantage; however, the anti-emetic effect can be a big advantage of using propofol.
Tables 3-
6 shows that desaturation and increasing O
2 supply are correlated with a lower dose of propofol (
P = 0.011,
P = 0.002). However, there is no correlation between using propofol and complications, such as desaturation and increasing oxygen supply (
P = 0.102,
P = 0.261). The complication portion of
Table 2 shows that desaturation and increasing O
2 supply occurred more in the midazolam + propofol group than in the propofol alone group. The propofol alone group showed no complications. The dose of propofol in the propofol alone group was higher than that in the combination group. Therefore, when the dose of propofol is low, desaturation and increasing O
2 supply appear more statistically significant. This does not mean that there are more complications in the low-dose propofol alone group than in the high-dose propofol alone group. Although contraindicated for use in patients under 3 years of age, it has recently been used for general anesthesia without difficulty.
22 Rajasekaran et al.
23 reported that the use of propofol alone yielded positive results in lowering complication rates in PSUE. Oh et al.
17 reported that the propofol group, compared with the midazolam group, demonstrated a faster recovery time and similar safety in 62 Korean children. An RCT study by Paspatis et al.
24 recommended the use of PO midazolam before propofol in children to reduce the effective dose of propofol and relieve its associated vascular pain. In this study, midazolam + propofol was the third most commonly used (206 cases, 28.1%). Although magnetic resonance imaging (MRI) and endoscopy are different procedures, a study that compared midazolam + ketamine and midazolam + propofol in pediatric MRI scans demonstrated that midazolam + ketamine enabled better quality MRI scans, which are easily affected by movement.
25 Midazolam + ketamine was also preferred over propofol in lumbar puncture.
26 However, in a large-scale literature review of PSUE, several limitations of midazolam or ketamine were found. A propofol-based sedation method was recommended in a meta-analysis study despite the shortage of RCT data.
2 In an RCT study by Khoshoo et al.,
27 it was established that propofol was superior to midazolam for use in children. In this study, all three drugs (midazolam, ketamine, propofol) were used in 18 cases (2.5%). Most were made by one unit in the institution where sedation and monitoring were performed by a pediatric anesthesiologist. Moreover, as mentioned earlier, the combination of the three drugs may offset the disadvantages of each drug, but it may also cause synergistic effects related to adverse events. The triple combination method requires experience in inducing deep sedation, as well as confidence in immediate treatment based on close monitoring.
The level and duration of procedural sedation should first be determined based on the type of procedure, after which the drug, administration route, dose, method and interval of administration, and combination with other drugs should be considered. During sedation, vital signs should be closely monitored to enable an immediate intervention for potential adverse events.
28 PSUE is defined as a high-anxiety and high-pain procedure in the Korean procedural sedation guidelines.
28 Based on this, general anesthesia or deep sedation is recommended, for instance by global PSUE guidelines, with a preference for IV ketamine. Midazolam with fentanyl, propofol with fentanyl, ketamine with propofol, and etomidate IV are recommended as alternatives.
28 Nonetheless, some drugs used by endoscopists have practical limitations. Endoscopy procedures interfere with airway maintenance. As the endoscope is inserted, it occupies or presses on the airway and mechanically blocks airflow. In addition, foreign-material stimulation from the tube causes excess secretion. These factors are important considerations in drug selection. A drug that does not cause respiratory difficulty or excess secretion or reduce such effects is well-suited for upper endoscopy. General anesthesia is not typically required for PSUE, although there may be slight differences between procedures. In this study, the average duration was 5.2 minutes in 651 cases (88.7%), with good observations of structures including the duodenum. There were 244 cases of biopsies of three or more sites (33.2%), 212 cases of biopsies of one or two sites (28.9%), and 278 cases with no biopsy (37.9%). Korean pediatric endoscopists are fully aware of such circumstances and apply appropriate sedation for their procedures. All 734 patients underwent successful PSUE without complications that required intubation. Shallow sedation with fewer potential adverse events was preferred over deep sedation for the convenience of the endoscopist. In this study, Korean pediatric endoscopists based their drug selections on the guidelines but opted for a slightly lower dose than recommended.
In conclusion, a summary of procedural sedation methods for Korean pediatric endoscopists is necessary, considering the specific characteristics of South Korea, pediatric sedation, and upper endoscopy. This study did not include all pediatric endoscopic units in Korea. There are limitations regarding the short period and the few patients involved. Although we tried to collect data as objectively as possible, the calculation of the Ramsey sedation score is subjective. We attempted to increase the accuracy by using data on the latest procedure. However, if the procedure sheet is not carefully filled, there is a high possibility that complications will be omitted. Despite the numerous limitations, this study is the first on Korean PSUE; however, PSUE has recently undergone significant developments. It cannot be established that one of the various methods implemented in South Korea is the best. The authors summarized the current state of sedation methods used in Korean pediatric endoscopy and the optimal guidelines to be considered. Furthermore, the authors intend to develop a pediatric endoscopist-directed sedative “recipe” for comfortable procedures with a low risk of adverse events through follow-up studies based on this study.
Go to :












 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download