1. Lau WK, Blute ML, Weaver AL, Torres VE, Zincke H. Matched comparison of radical nephrectomy vs nephron-sparing surgery in patients with unilateral renal cell carcinoma and a normal contralateral kidney. Mayo Clin Proc. 2000; 75(12):1236–1242. PMID:
11126830.

2. Lee JW, Kim H, Choo M, Park YH, Ku JH, Kim HH, et al. Different methods of hilar clamping during partial nephrectomy: impact on renal function. Int J Urol. 2014; 21(3):232–236. PMID:
24033600.

3. Leibovich BC, Blute M, Cheville JC, Lohse CM, Weaver AL, Zincke H. Nephron sparing surgery for appropriately selected renal cell carcinoma between 4 and 7 cm results in outcome similar to radical nephrectomy. J Urol. 2004; 171(3):1066–1070. PMID:
14767272.

4. Patard JJ, Pantuck AJ, Crepel M, Lam JS, Bellec L, Albouy B, et al. Morbidity and clinical outcome of nephron-sparing surgery in relation to tumour size and indication. Eur Urol. 2007; 52(1):148–154. PMID:
17240036.

5. Li W, Cheng Y, Cheng Y, Ren H, Han N. Clinical efficacy of radical nephrectomy versus nephron-sparing surgery on localized renal cell carcinoma. Eur J Med Res. 2014; 19(1):58. PMID:
25374003.

6. Tufek I, Mourmouris P, Doganca T, Obek C, Argun OB, Tuna MB, et al. Robot-assisted partial nephrectomy for T1b tumors: strict trifecta outcomes. JSLS. 2017; 21(1):e2016.00113.

7. Boulière F, Crepel M, Bigot P, Pignot G, Bessede T, de la Taille A, et al. Nephron-sparing surgery is superior to radical nephrectomy in preserving renal function outcome in tumors larger than 4 cm. Prog Urol. 2011; 21(12):842–850. PMID:
22035910.
8. Crépel M, Jeldres C, Perrotte P, Capitanio U, Isbarn H, Shariat SF, et al. Nephron-sparing surgery is equally effective to radical nephrectomy for T1BN0M0 renal cell carcinoma: a population-based assessment. Urology. 2010; 75(2):271–275. PMID:
19962740.

9. Peyronnet B, Seisen T, Oger E, Vaessen C, Grassano Y, Benoit T, et al. Comparison of 1800 robotic and open partial nephrectomies for renal tumors. Ann Surg Oncol. 2016; 23(13):4277–4283. PMID:
27411552.

10. Benway BM, Bhayani SB, Rogers CG, Dulabon LM, Patel MN, Lipkin M, et al. Robot assisted partial nephrectomy versus laparoscopic partial nephrectomy for renal tumors: a multi-institutional analysis of perioperative outcomes. J Urol. 2009; 182(3):866–872. PMID:
19616229.

11. Mottrie A, Borghesi M, Ficarra V. Is traditional laparoscopy the real competitor of robot-assisted partial nephrectomy? Eur Urol. 2012; 62(6):1034–1036. PMID:
22854247.

12. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009; 150(9):604–612. PMID:
19414839.

13. Hafez KS, Fergany AF, Novick AC. Nephron sparing surgery for localized renal cell carcinoma: impact of tumor size on patient survival, tumor recurrence and TNM staging. J Urol. 1999; 162(6):1930–1933. PMID:
10569540.

14. Antonelli A, Cozzoli A, Nicolai M, Zani D, Zanotelli T, Perucchini L, et al. Nephron-sparing surgery versus radical nephrectomy in the treatment of intracapsular renal cell carcinoma up to 7cm. Eur Urol. 2008; 53(4):803–809. PMID:
18036730.

15. Pahernik S, Roos F, Röhrig B, Wiesner C, Thüroff JW. Elective nephron sparing surgery for renal cell carcinoma larger than 4 cm. J Urol. 2008; 179(1):71–74. PMID:
17997423.

16. Shum CF, Bahler CD, Sundaram CP. Matched comparison between partial nephrectomy and radical nephrectomy for T2 N0 M0 tumors, a study based on the National Cancer Database. J Endourol. 2017; 31(8):800–805. PMID:
28486848.

17. Ficarra V, Bhayani S, Porter J, Buffi N, Lee R, Cestari A, et al. Predictors of warm ischemia time and perioperative complications in a multicenter, international series of robot-assisted partial nephrectomy. Eur Urol. 2012; 61(2):395–402. PMID:
22079308.

18. Patel HD, Mullins JK, Pierorazio PM, Jayram G, Cohen JE, Matlaga BR, et al. Trends in renal surgery: robotic technology is associated with increased use of partial nephrectomy. J Urol. 2013; 189(4):1229–1235. PMID:
23085300.

19. Hanzly M, Frederick A, Creighton T, Atwood K, Mehedint D, Kauffman EC, et al. Learning curves for robot-assisted and laparoscopic partial nephrectomy. J Endourol. 2015; 29(3):297–303. PMID:
25111313.

20. Shen Z, Xie L, Xie W, Hu H, Chen T, Xing C, et al. The comparison of perioperative outcomes of robot-assisted and open partial nephrectomy: a systematic review and meta-analysis. World J Surg Oncol. 2016; 14(1):220. PMID:
27549155.

21. Xia L, Wang X, Xu T, Guzzo TJ. Systematic review and meta-analysis of comparative studies reporting perioperative outcomes of robot-assisted partial nephrectomy versus open partial nephrectomy. J Endourol. 2017; 31(9):893–909. PMID:
27305835.

22. Godoy G, Ramanathan V, Kanofsky JA, O'Malley RL, Tareen BU, Taneja SS, et al. Effect of warm ischemia time during laparoscopic partial nephrectomy on early postoperative glomerular filtration rate. J Urol. 2009; 181(6):2438–2443. PMID:
19371905.

23. Thompson RH, Lane BR, Lohse CM, Leibovich BC, Fergany A, Frank I, et al. Every minute counts when the renal hilum is clamped during partial nephrectomy. Eur Urol. 2010; 58(3):340–345. PMID:
20825756.

24. Lee C, Kwon T, Yoo S, Jung J, Lee C, You D, et al. Comparison of renal function between robot-assisted and open partial nephrectomy as determined by Tc 99m-DTPA renal scintigraphy. J Korean Med Sci. 2016; 31(5):743–749. PMID:
27134496.

25. Simmons MN, Fergany AF, Campbell SC. Effect of parenchymal volume preservation on kidney function after partial nephrectomy. J Urol. 2011; 186(2):405–410. PMID:
21680004.

26. Simmons MN, Lieser GC, Fergany AF, Kaouk J, Campbell SC. Association between warm ischemia time and renal parenchymal atrophy after partial nephrectomy. J Urol. 2013; 189(5):1638–1642. PMID:
23159462.





 PDF
PDF Citation
Citation Print
Print



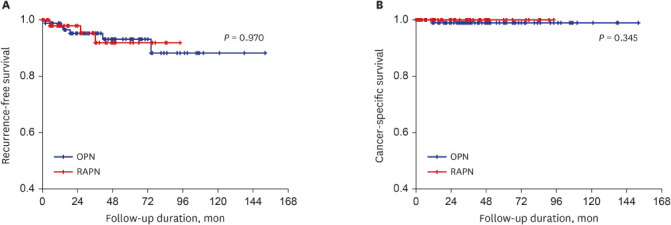
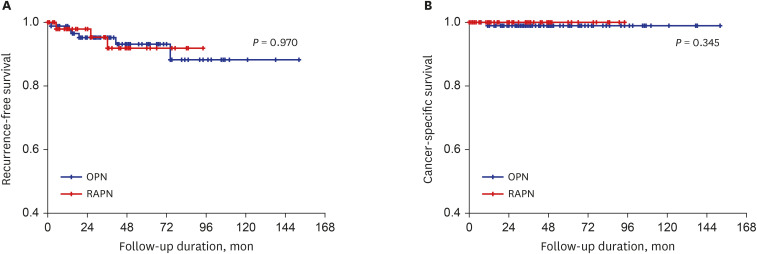
 XML Download
XML Download