INTRODUCTION
Endovascular therapy (EVT) has been widely applied in the treatment of peripheral artery diseases (PADs) due to its minimal invasiveness and lower perioperative morbidity compared with surgical treatment.
1)2)3) Atherosclerotic aortoiliac disease is commonly observed in patients undergoing EVT for symptomatic lower extremity artery disease.
4) In a recent guideline, EVT was recommended as a first-line treatment strategy not only for short aortoiliac lesions but also for extensive lesions in patients with severe comorbidities.
3) Although both primary and provisional stenting may achieve similar results in patients with limited aortoiliac lesions, primary stenting may provide superior outcomes for complex aortoiliac lesions.
3)5) Currently, several different types of stents such as self-expanding, balloon-expanding, and covered stents are available for the treatment of aortoiliac lesions. However, there is limited information comparing the effectiveness of these devices.
6) Iliac artery lesions tend to be tortuous and calcified. In particular, distal external iliac artery lesions are exposed to forces related to the movement of the hip joint.
7) The EPIC™ stent (Boston Scientific, Natick, MA, USA) is a self-expanding nitinol stent with a hybrid design of open and closed cell structures that may enhance its flexibility and conformational dilation capability.
8) The purpose of the present study was to investigate the clinical efficacy and safety of the EPIC™ stent in the treatment of iliac artery diseases using a Korean multicenter prospective registry (K-EPIC Registry).
METHODS
Study population and enrollment
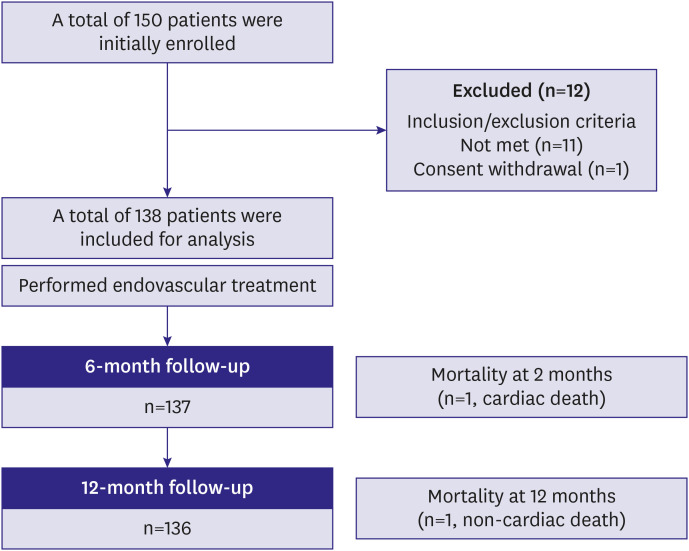
This study was a single-arm, prospective, multicenter trial evaluating the EPIC™ self-expanding nitinol stent in the treatment of atherosclerotic iliac artery diseases. The trial was conducted in 9 sites in South Korea following the Declaration of Helsinki 2013. The study protocol was approved by the Institutional Review Board (IRB) of Yonsei University Health System (IRB number: 1-2015-0075). All patients provided written informed consent before enrollment.
Patients were eligible if all following inclusion criteria were met, such as age ≥19 years, clinical presentation of Rutherford category 2 to 4, stenotic (≥50% diameter stenosis) or occlusive iliac artery lesions, and an ankle-brachial index (ABI) <0.9. Exclusion criteria included acute severe limb ischemia, severe critical limb ischemia of Rutherford category 5 or 6, age >85 years, known allergic reactions to heparin, aspirin, clopidogrel, or contrast agent, in-stent restenosis, previous bypass surgery in the targeted vessel, uncontrolled congestive heart failure or left ventricular ejection fraction ≤40%, severe hepatic dysfunction (aspartate transaminase or alanine aminotransferase ≥3-fold higher than the normal reference value), significant leukopenia, neutropenia, thrombocytopenia, anemia, or risk of bleeding, pregnant or fertile women, life expectancy under 1 year, and non-treated significant inflow disease. Before enrollment, each patient's medical history, baseline medications, Rutherford classification, Korean-Peripheral Artery Questionnaire (K-PAQ)
9) for the investigation of quality of life, ABI, at least one imaging study such as computed tomography (CT), magnetic resonance imaging, duplex ultrasound, or catheter angiography and Trans-Atlantic Inter-Society Consensus (TASC) II type assessment, laboratory tests including blood cell count, routine chemistry, lipid profile, and C-reactive protein were performed.
K-PAQ is the Korean version of the Peripheral Artery Questionnaire of 20-item health status used for patients with PAD. Each question asks about symptoms related to PAD over the previous 4 weeks. The questionnaire consists of 6 domains (symptoms, symptom stability, physical limitations, treatment satisfaction, social functioning, and quality of life) with scored responses. A summary score represents the average score for symptoms, physical limitation, social functioning, and quality of life. The scores range from 0 to 100, where higher scores represent better control of symptoms, reduced physical limitation, better treatment satisfaction and social functioning, and better quality of life.
Endovascular and therapy
Before the procedure, patients were pre-medicated with aspirin (300 mg, at least 24 hours prior) and clopidogrel (300 mg, at least 12 hours prior) if patients had not taken aspirin and clopidogrel for at least 5 days before the procedure. After the insertion of the introducer sheath, a bolus of 5,000 units of unfractionated heparin was administrated and activated coagulation time was maintained above 250 seconds.
Vascular access to the target lesion was obtained by either the ipsilateral retrograde or the contralateral cross-over approach from the common femoral artery, using a percutaneous method. An antegrade approach via a brachial or radial artery was also permitted if necessary. A 6–7 F sheath (TERUMO Inc., Tokyo, Japan) was used on the ipsilateral approach and a 6–7 F sheath (Balkin or Ansel; Cook Inc., Bloomington, IN, USA) was used on the contralateral approach. To cross the target lesion, a 0.018 or 0.035-inch guidewire was used. To evaluate the characteristics of the iliac artery lesion and runoff vessels, pre-procedure angiography was obtained from the proximal iliac artery to the distal tibial artery. In cases of complete occlusion, an intraluminal or subintimal approach was selected at the operator's discretion. Target lesions were pre-dilated with a balloon catheter to a diameter equal-to or smaller-than the reference diameter. All iliac target lesions were treated with EPIC™ stents. The stent diameter was chosen to be 1 mm larger than the target lesion reference diameter. The stent length was selected to be sufficient to cover the whole diseased segment. Post-dilation was performed with a balloon of vessel diameter at a relatively low inflation pressure. After EVT, dual antiplatelet agents (aspirin at 100 mg/day and clopidogrel at 75 mg/day) were administrated for at least 6 months. After 6 months, single antiplatelet therapy with aspirin or clopidogrel was maintained.
Follow-up
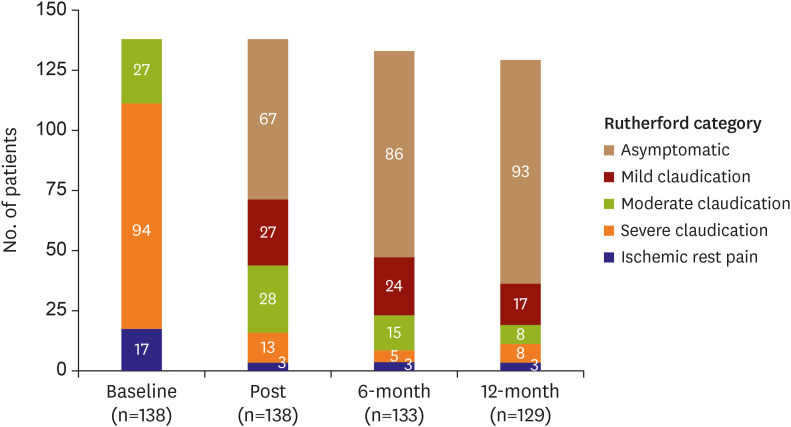
After EVT, patients were evaluated for symptom status via Rutherford classification and ABI, and laboratory tests were performed. Patients were followed-up routinely at 1 month after the procedure and 3- or 6-month intervals thereafter. At each follow-up, clinical evaluations including Rutherford classification and physical examinations were performed. ABI was repeated at 6 months and 1 year after the procedure, or at any time if symptom status deteriorated. K-PAQ was performed at the 1-month and 1-year follow-up. An imaging evaluation such as CT angiography, duplex ultrasound, or intra-arterial angiography was recommended at the 1-year follow-up or at any time if the patient's symptoms worsened by ≥1 Rutherford category or if symptom status deteriorated and ABI decreased by >0.15.
Endpoints and definitions
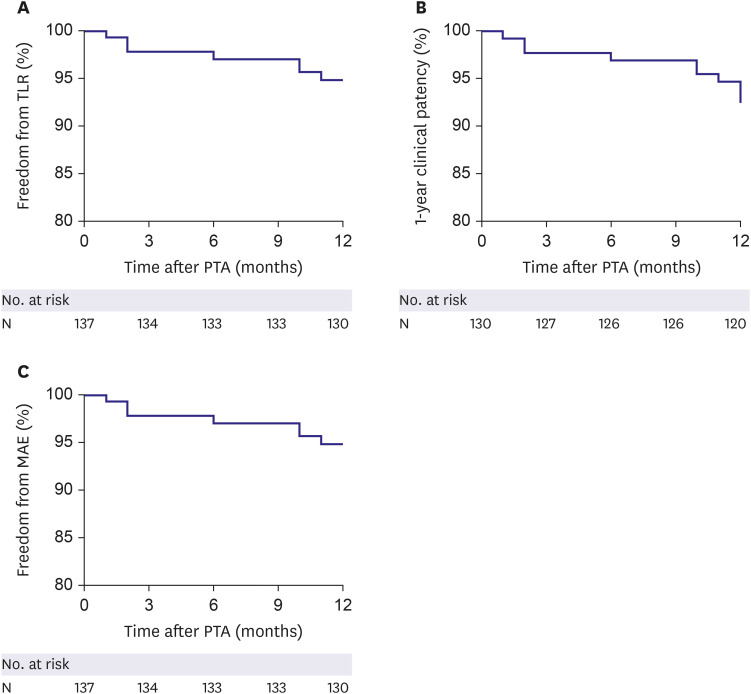
The primary endpoint was the 1-year freedom from target lesion revascularization (TLR), which was defined as any surgical or percutaneous intervention to the target lesion after initial EVT due to restenosis of the target lesion with worsened symptoms. The secondary endpoints were a 1-year clinical patency rate and freedom from major adverse events (MAEs) after implantation of EPIC™ stents. Clinical patency was defined as freedom from symptom aggravation by increased Rutherford category combined with a decrease in ABI over 0.15 compared with post-procedure ABI or as an absence of restenosis (>50%) on imaging modalities, such as duplex ultrasound, angiography, or CT based angiography. MAE was defined as a composite of procedure-related death within one month, in-hospital myocardial infarction, TLR, and amputation of the treated limb. Procedural success was defined as the absence of residual stenosis more than 30% or arterial dissection limiting blood flow.
Statistical analysis
Continuous variables are presented as the mean±standard deviation, and categorical variables are presented as frequency and percentage. Baseline clinical and lesion characteristics were analyzed using the Student's t-test for continuous data and χ2 and Fisher's exact test for categorical data. The one-way analysis of variance was used for comparisons of K-PAQ scores at baseline, 1 month, and 1 year of follow-up.
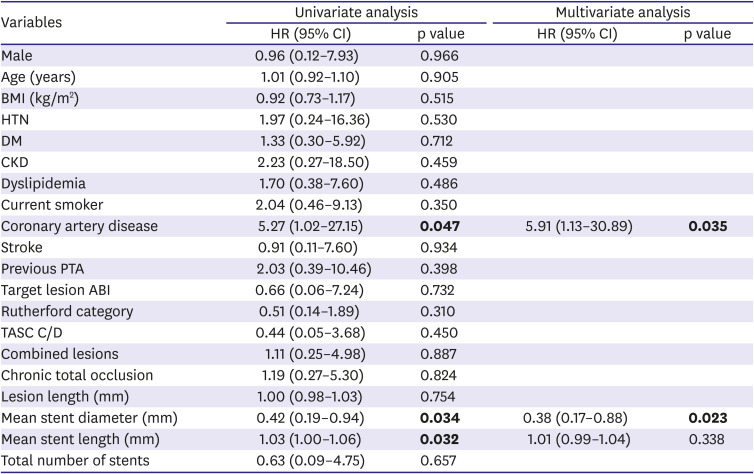
Freedom from TLR, clinical patency, and MAE were assessed using the Kaplan-Meier method and a log-rank test was used to compare each group. The predictors of TLR were evaluated using a univariate and multivariate analyses of the Cox proportional hazard regression. All variables with p<0.15 in univariate analysis were included in the multivariate analysis model.
A p value <0.05 was considered significant. All statistical analyses were performed using SPSS 25.0 software (IBM Corp., Armonk, NY, USA).
DISCUSSION
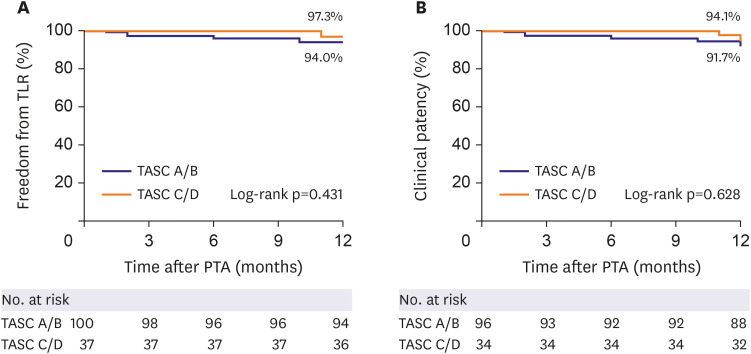
This prospective registry study evaluated clinical outcomes in patients treated with EPIC™ self-expanding stents for iliac lesions and evaluated risk factors to predict TLR. The main findings of the present study were as follows: 1) The EPIC™ stent showed excellent clinical outcomes in terms of freedom from TLR (94.9%) and clinical patency (92.3%) at 1-year of follow-up. 2) The incidence of procedure-related complications was rare (1.4%). 3) The 1-year rate of MAE also remained low (5.1%). 4) In our study population, combined coronary artery disease and smaller stent diameter were identified as independent risk factors of TLR after EPIC™ stent implantation in the iliac artery lesions. 5) The lesion complexity, such as TASC C/D, had no apparent impact on patency after EVT.
In a prior randomized trial, the first generation nitinol stent (SMART™; Cordis, Warren, NJ, USA) demonstrated comparable primary patency (94.7% vs. 94.6%) in iliac lesions at 12 months compared with Wallstent™, a braided Elgiloy stainless steel stent.
10) However, the procedural success rate of the SMART™ stent was significantly higher than that of the Wallstent™, at 98.2% and 87.5%, respectively. Owing to the characteristic properties of nitinol, such as shape memory and superelasticity,
11) self-expanding nitinol stents appear to be effective for maintaining patency in complex aortoiliac lesions by preventing vessel collapse and ensuring stability. Other studies evaluating nitinol stents such as Protégé stents (Medtronic, Plymouth, MN, USA) and the Astron stent (Biotronik AG, Bülach, Switzerland) have also reported favorable outcomes in the treatment of iliac artery disease. The 9-month primary patency and freedom from the target for Protégé stents in the DURABILITY study were 95.8% and 98.6%, respectively. The 12-month primary patency and freedom from TLR for the Astron stent were 89.8% and 97.9%, respectively.
12) The EPIC™ stent is a self-expanding nitinol stent with a hybrid design of open- and closed-cell geometry engineered to provide flexibility. The efficacy and safety of the EPIC™ stent have been previously evaluated in the ORION trial, a single-arm prospective multicenter clinical trial, which enrolled 125 patients with 166 iliac artery lesions in 28 sites in the USA. At the 12-month follow-up, the freedom from the TLR rate was 94.6% and the primary patency was 94.4%.
13) A Japanese multicenter retrospective study also investigated the clinical outcomes of the EPIC™ stent in aortoiliac artery disease and reported similar 12-month rates of freedom from TLR (96.0%) and primary patency (92.2%).
8) Our study demonstrated results that were consistent with those previous studies. Similarly to our study, the ORION study did not show a significant difference in the primary patency rate between the TASC A/B group vs. TASC C/D group (96.1% and 97.6%).
13)
The efficacy of self-expanding stents was shown in a randomized controlled study comparing self-expanding stents vs. balloon-expandable stents for iliac artery disease.
14) In this study, the self-expanding stents showed superior outcomes in terms of primary patency (94.5% vs. 87.0%) and freedom from TLR (97.2% vs. 93.6%) relative to balloon-expandable stents.
Predictable factors of revascularization after the implantation of nitinol stents in iliac artery lesions have been analyzed in several studies. Tsujimura et al.
8) reported that diabetes mellitus and a small reference vessel diameter were predictors of lesion patency. In the BRAVISSIMO study,
15) kissing stent technique and obesity were independent risk factors for restenosis. Interestingly, Bechter-Hugl et al.
16) found that younger age, especially in female patients, was a significant predictor of restenosis, as was a smaller stent diameter. Our study also found that a small stent diameter (HR, 0.38; p=0.023) was an independent risk factor for TLR. Furthermore, coronary artery disease was another independent risk factor for TLR (HR, 5.91; p=0.035) in our study. The reason for the significant association between coronary artery disease and the development of restenosis after iliac stenting remains unclear. It is possible that patients with polyvascular disease, involving more than one vascular system, would have more progressed atherosclerosis than PAD alone, and would be high-risk patients to develop restenosis after revascularization. Previous studies have shown that polyvascular atherosclerotic disease in PAD patients is associated with increased rates of mortality, major adverse cardiac events, and lower extremity revascularization.
17)18)
This study had several limitations. First, the study population was relatively small. In particular, subjects with complex iliac artery lesions such as TASC C or D were not sufficiently represented. Second, follow-up imaging studies such as CT angiography, vascular angiography, or Doppler ultrasound were not consistently performed in all patients, potentially contributing to bias. We defined primary clinical patency as freedom from symptom aggravation with an ABI decrease >0.15 or absence of restenosis on imaging studies. Thus, restenosis without causing an ABI decrease >0.15 or related symptoms may have been undetected, and the primary patency rate may have been overestimated. Third, the duration of clinical follow-up in this study was only one year, which may be not sufficient to evaluate the efficacy of stents in iliac arteries.
In conclusion, in a multicenter prospective registry study, the EPIC™ stent demonstrated excellent immediate and 1-year efficacy and safety in patients with iliac artery disease.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download