INTRODUCTION
Atrial fibrillation (AF) is the most common cardiac arrhythmia, and stroke prevention with oral anticoagulation (OAC) is central to its management when stroke risk factors are present.
1) With the advent of the non-vitamin K antagonist oral anticoagulants (NOACs), the perspective for stroke prevention in AF has changed.
2) Coronary artery disease (CAD) is reported in 20%–40% of AF patients.
3) Over the lifetime course, 5–15% of AF patients are known to receive percutaneous coronary intervention (PCI), and 5–10% of PCI patients have concomitant AF.
4) Although OAC therapy is recommended to reduce the risk of ischemic stroke in patients with AF, dual antiplatelet therapy (DAPT) is recommended to reduce the risk of stent thrombosis in patients undergoing PCI. However, such a combination of antiplatelet agents and antithrombotic therapy with OAC may increase the risk of bleeding.
Recently, major clinical trials have reported the benefits of NOAC-based double antithrombotic therapy (DAT) over warfarin-based triple antithrombotic therapy (TAT) in AF patients with CAD undergoing PCI.
5)6)7)8) Hence, international guidelines have updated their recommendations based on these trials.
1)9)10)11)
Despite the rapidly evolving evidence-based strategies of antithrombotic therapy in AF patients with PCI, appropriate antithrombotic regimens are suboptimally prescribed in real-world practice, specifically in Asia. A previous study reported high rates of antiplatelet use and underutilization of OAC use in Korean AF patients after PCI.
12) One possible reason is that Asian patients with AF are known to have higher risks of stroke and bleeding than non-Asian patients.
13) Also, Asians are perceived to have a higher risk of bleeding for both antiplatelet agents and oral anticoagulants compared to non-Asians.
14) In the REDUAL-PCI trial, the Japanese subgroup had a higher rate of International Society on Thrombosis and Haemostasis major or clinically relevant non-major bleeding, while a lower rate of myocardial infarction (MI) or stent thrombosis than the overall population.
15) Therefore, there is the need to investigate the status of antithrombotic therapy in ‘real-world’ practice in Asian AF patients treated with PCI.
This study aimed to investigate how periprocedural antithrombotic regimens have been changed since the introduction of NOACs among Korean patients with AF undergoing PCI.
METHODS
This was a retrospective cross-sectional study using the claims database from the Health Insurance Review and Assessment (HIRA) during 2013–2018. The HIRA database provides medical claims information on the entire Korean population.
16) Its database comprises not only comprehensive claims information on prescriptions and procedures but also demographic data of each insured member. The HIRA database also has the disease status of each member encoded in the International Classification of Disease, Tenth Revision, Clinical Modification.
16) The data can be provided to researchers for academic purposes upon request. This study was approved by the Seoul National University Hospital Institutional Review Board (IRB) (IRB No. E-1911-052-1078), and the informed consent was waived by the review board due to the anonymized characteristics of the data. The study was conducted according to the ethical guidelines of the Declaration of Helsinki revised in 2013.
Study population and definitions
From the claims database, we identified the patients with AF undergoing PCI during 2013–2018. We used well-defined operational definitions for AF and PCI validated in a previous study.
16) AF was defined as having diagnostic codes of I48.0-48.4 or I48.9 during hospitalization or outpatient clinics. PCI was defined as having procedural codes of M6551, M6552, M6561-6564, M6571, and M6572. Study subjects were defined as those who had AF before PCI. Finally, we excluded patients with mitral stenosis (I50, I52, and I59) or prosthetic heart valves (Z952-Z954).
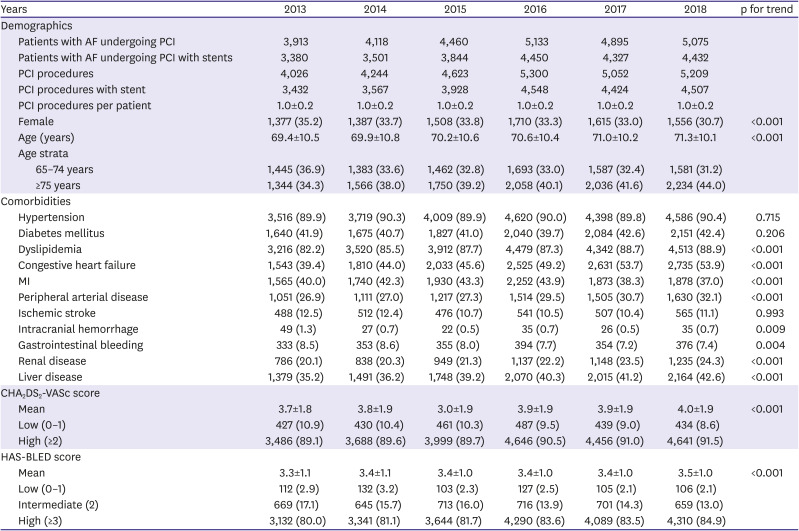
Using diagnostic codes, we defined several study variables of comorbidities, including hypertension, diabetes mellitus, dyslipidemia, peripheral arterial disease, ischemic stroke, systemic thromboembolism, intracranial hemorrhage, gastrointestinal bleeding, renal disease, and liver disease.
16) Their details are presented in
Supplementary Table 1. To evaluate the risks of ischemic stroke and bleeding, we used the CHA
2DS
2-VASc and HAS-BLED scores, respectively.
17) HAS-BLED scores were calculated without international normalized ratio, and the amount of alcohol consumption as such information was not available in the HIRA database.
2) High risk of ischemic stroke or bleeding was defined as a CHA
2DS
2-VASc score ≥2 or HAS-BLED score ≥3, respectively.
Antithrombotic regimens
We examined the claims data for antithrombotic agents prescribed outpatient or inpatient for each patient. For antithrombotic agents, we included aspirin, clopidogrel, prasugrel, ticagrelor, warfarin, and NOAC (dabigatran, rivaroxaban, apixaban, and edoxaban). In Korea, NOAC was fully reimbursed after July 2015; thus, the prescription of NOAC has dramatically increased since then. The antithrombotic regimen was evaluated within 30 days after receiving PCI. The prescription period of interest was limited in the present study because this study aimed to examine the pattern of the management for antithrombotic therapy early after PCI.
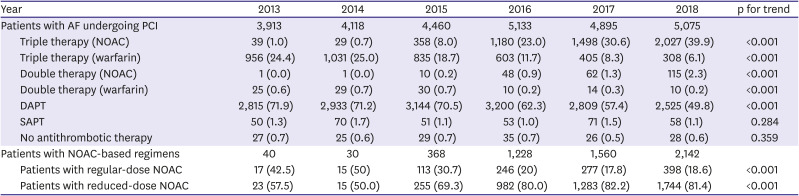
We classified antithrombotic regimens according to the combination of prescribed agents into single antiplatelet therapy, DAPT, DAT (warfarin or NOAC-based), and TAT (warfarin or NOAC-based). Meanwhile, multiple combinations of antithrombotic prescriptions may be present in a patient within 30 days of PCI, in which case, the prescription comprising the maximum combination was selected.
Statistical analyses
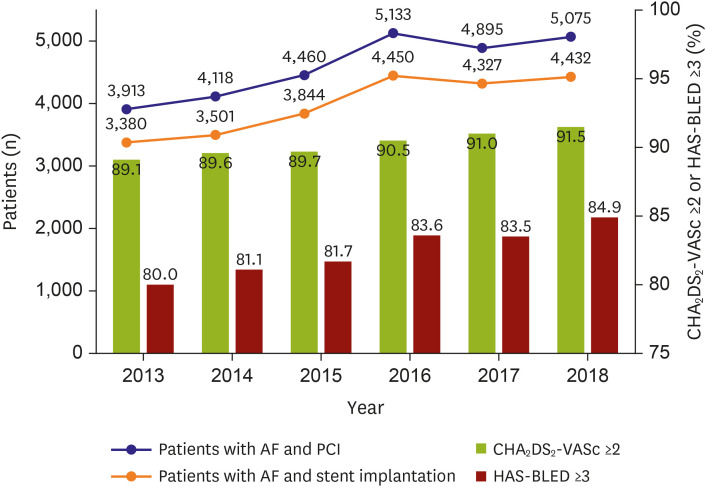
The annual number of patients with AF undergoing PCI was investigated. The baseline characteristics, including age, sex, comorbidities, CHA
2DS
2-VASc, and HAS-BLED scores of the annual population, were evaluated. Data are presented as number (%) for dichotomous or categorical variables and as mean±standard deviation for continuous variables. The significance of a temporal trend of each study variable was analyzed using linear regression analysis over the study period. To investigate whether there were significant interactions between antithrombotic regimens and subgroups, we performed subgroup analyses for sex, high risks of ischemic stroke, and bleeding. We also performed a subgroup analysis for those hospitalized due to MI. MI was defined by having a primary diagnostic code of either I21 or I22 for hospitalization. This operational definition of MI has been validated to have a positive predictive value of 92% in our previous study.
18) To evaluate the independent predictors of favoring TAT over DAPT, we performed multivariate logistic regression analysis. Moreover, multivariate logistic regression analysis was performed to investigate the differences in the preference of TAT over DAPT across the scores of CHA
2DS
2-VASc or HAS-BLED. All analyses were considered as statistically significant if the two-tailed p-value was less than 0.05. All statistical analyses were performed using the Statistical Analysis System (SAS) version 9.3 (SAS Institute Inc., Cary, NC, USA).
DISCUSSION
This study investigated the trends of periprocedural antithrombotic therapy in patients with AF undergoing PCI using the claims database in the NOAC era. Our principal findings are as follows: (i) the number of Korean patients with AF undergoing PCI has been substantially increased after the introduction of NOACs in 2013; (ii) the risk profile for both ischemic stroke and bleeding of these patients has been increasing annually; (iii) periprocedural antithrombotic regimen has shifted from DAPT-based to NOAC-based TAT, but DAPT was still the most favored antithrombotic therapy; (iv) the predictors of underuse of TAT were intracranial hemorrhage, renal disease, dyslipidemia, MI, peripheral arterial disease, and female sex; and (v) TAT or DAPT was seemed to be preferred in the case of a high risk of ischemic stroke or bleeding, respectively, although this trend was not statistically significant.
This study has the following main strengths: it evaluated AF-PCI patients on a national scale and, to the best of our knowledge, included the largest number of patients. The management of AF-PCI patients has been rapidly evolving in recent years as NOACs were introduced, but detailed temporal trend data on real-world clinical practice have been relatively rare. This study illustrates a clear gap between guideline recommendations and real-world clinical practice.
Before 2016, studies on antithrombotic therapy in AF-PCI patients had investigated the combination therapy of warfarin and antiplatelet drugs. The WOEST trial
19) compared bleeding events 1 year after PCI between the TAT group treated with warfarin, aspirin, and clopidogrel and the DAT group treated with warfarin and clopidogrel. This trial found that compared to TAT, DAT significantly reduced the risk of bleeding without increasing the risk of thrombotic events. A Danish nationwide cohort study reported that warfarin-based DAT showed a lower risk of bleeding than TAT, without increasing the risk of ischemic events.
20)
Since 2016, studies have been focused on using combination therapy of NOAC and antiplatelet drugs. Clinical trials, including PIONEER AF-PCI,
5) RE-DUAL PCI,
6) AUGUSTUS,
7) and ENTRUST-AF-PCI,
8) studied DAT based on rivaroxaban, dabigatran, apixaban, and edoxaban, respectively. Although the study designs were different, NOAC-based DAT was associated with a lower risk of bleeding without increasing the risk of ischemic events compared to warfarin-based TAT.
21)22) Accordingly, various international guidelines have been updated.
1)9)10)11)
All the guidelines largely advocate approaches that have more similarities than differences. First, a thorough evaluation of the risks of the ischemic events and bleeding is needed before the decision of an antithrombotic regimen. Second, NOACs are generally preferred to warfarin if anticoagulant therapy is required among AF patients with PCI. Third, in the periprocedural period, the combination of aspirin and clopidogrel is usually recommended, regardless of treatment strategy, provided that the risk of bleeding is not significantly high. Therefore, when AF patients requiring OAC receive PCI, both North American and European guidelines recommend NOAC-based TAT during the periprocedural period. Depending on the balance between the risk of ischemic events and bleeding, the period of TAT needed to be adjusted from 1 to 6 months individually. Afterward, NOAC-based DAT up to 12 months followed by NOAC monotherapy is recommended.
21)
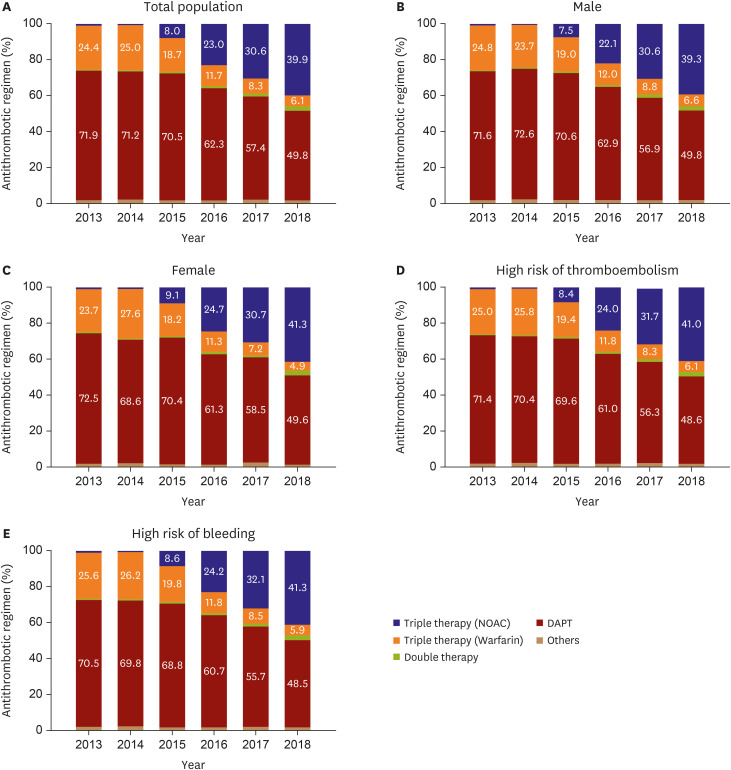
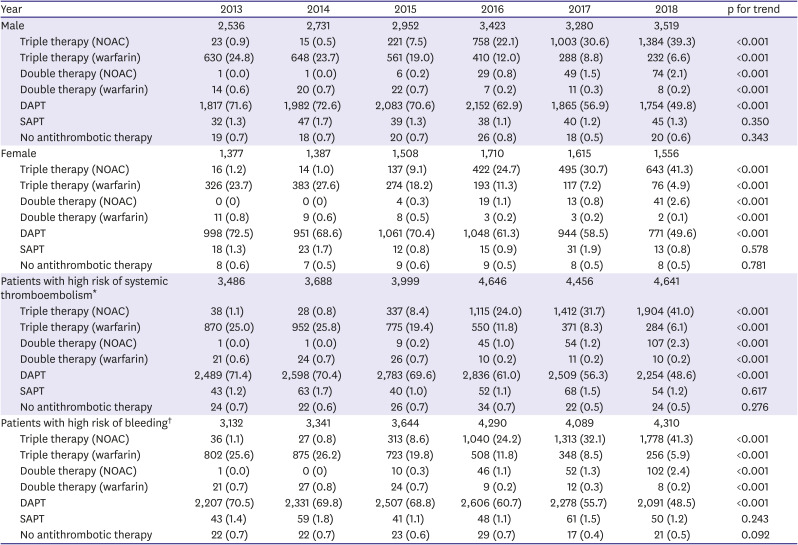
In this study, the proportion of patients receiving DAT was significantly small (<3%), and most received TAT or DAPT. We investigated which factors favored TAT or DAPT. Importantly, a high risk of bleeding (HAS-BLED score ≥3) did not have a significant association with the treatment choice, although a high HAS-BLED score should be used to identify and mitigate reversible bleeding risk factors and to identify high-risk patients for early review and follow-up.
23) In contrast, a high risk of thromboembolism (CHA
2DS
2-VASc scores ≥2) was associated with TAT (
Figure 3). The most important factor favoring DAPT was a history of intracranial hemorrhage, which was presumed to be due to the recurrence of hemorrhage, although DAPT has a similar risk of ICH as OAC.
24) We found that DAPT was preferred if the patient had intracranial hemorrhage, renal disease, dyslipidemia, or vascular diseases, such as MI and peripheral arterial disease. We assume that more attention tends to be focused on the management of MI rather than stroke prevention in patients with AF who receive PCI for acute MI. Briefly, stroke risk factors favored TAT, whereas the covariates specific to the risk of bleeding favored DAPT. The covariates common to the risks of both stroke and bleeding favored TAT.
Non-Asians have a lower risk of major bleeding on administration of oral anticoagulants than Asians.
25) Even in the Western populations, OACs in AF patients after PCI are underprescribed. In the CRUSADE registry of 1,648 patients with non-ST segment elevation MI and AF, only 27% were prescribed TAT, whereas 73% were prescribed DAPT at discharge.
26) Such a suboptimal usage of OACs is considered to be affected by concerns of bleeding when prescribing TAT, specifically in elderly individuals. In another Danish study analyzing 12,165 AF patients with PCI from 2001 to 2009, the proportion of TAT was only accounted for in 15.6%.
27)
The real-world data of non-Asians have been reported on antithrombotic therapy in patients with AF undergoing PCI.
28) In the Danish nationwide registries from 2011 to 2017, NOAC with DAPT had a lower risk of bleeding than warfarin with DAPT, with a comparable risk of thromboembolism. Another Danish nationwide registries study from 2011 to 2016 found that the proportion of TAT had been increased (43% in 2011 to 60% in 2016), while that of DAT decreased.
29) After the introduction of NOACs, warfarin-based therapy decreased, while NOAC-based therapy increased. Although DAPT was the most common antithrombotic regimen, this study did not provide the temporal trend of DAPT during the study period.
Consistent with the Danish study,
28) we found that NOAC-based therapy outpaced warfarin-based therapy from 2016, which was related to changes in the reimbursement criteria in Korea. Moreover, prescriptions of TAT increased, and those of DAPT decreased, but DAPT was the most preferred antithrombotic regimen in patients with AF undergoing PCI. Based on these studies, stroke prevention using OAC remains underutilized in AF patients with PCI among both Asian and non-Asian populations.
In Asia, OACs are more suboptimally prescribed in patients with AF undergoing PCI. One study investigated the temporal trends of antithrombotic therapy in Korean AF-PCI patients during 2006 and 2015, whereby the proportion of TAT increased from 22.7% to 38.2%, but DAPT still comprised the largest proportion at 60.3%.
12) However, there is a caveat that this study examined the antithrombotic regimen over the entire year of PCI, unlike the current study, which investigated only the regimen during the periprocedural period (i.e., within 30 days after PCI). One real-world study from Taiwan also showed that DAPT was the most common regimen (72.0%), while TAT was used in only 15.0% of patients.
30) Thus, despite several international guidelines that recommend NOAC-based TAT during the periprocedural period for AF-PCI patients, adherence to guidelines using OAC is still suboptimal despite the introduction of NOACs. The underuse of OACs appears to be due to concerns that adding warfarin to DAPT may increase the risk of bleeding, while the benefit of reducing thromboembolism is unclear among Asians. Since NOACs are rapidly replacing warfarin with better efficacy and safety for stroke prevention in patients with AF, there is the need to reconsider OAC-based therapy over DAPT in line with the recent evidences and guidelines. Meanwhile, we also found that most of NOACs were prescribed as reduced-dose (81.4% of total NOAC-based regimens in 2018) (
Table 2). This pattern might reflect the concerns of bleeding for using triple therapy with regular-dose NOACs.
This study shares the limitations of other studies based on claims data. First, there may be a discrepancy between the prescriptions and the medications actually consumed by patients, and such a difference cannot be further evaluated in this study. For a given individual, the antithrombotic prescription chosen in this study was the one with the maximum combination of antiplatelet or anticoagulant drugs within 30 days after the PCI. Therefore, if OACs were prescribed prior to PCI, DAT or TAT may have been underestimated. Second, since this study used claims data, which lack detailed clinical information or medical records, we could not investigate the reasons for patients with DAPT not being prescribed OACs. Third, because this study is a cross-sectional observational study, it is not possible to confirm how the clinical outcome differs for each regimen. Fourth, the operational definition of the maximum combination of antithrombotic therapy might influence on the proportion of DAT. We analyzed the maximum combination of antithrombotic drugs prescribed within 30 days after PCI. Those who had changed from TAT to DAT within 30 days after PCI were regarded as the TAT group. Therefore, there is a chance that TAT may be over-estimated, whereas DAT might be under-estimated. If we consider a more extended period after one month from PCI, a higher proportion of DAT would be expected. This study focused on the treatment during the periprocedural period, and long-term therapy should be analyzed in a follow-up study. Lastly, it is challenging to estimate the duration of TAT accurately because of some differences in the date of claiming the reimbursement and prescription date.
After the introduction of NOACs for stroke prevention in AF, early periprocedural antithrombotic regimens in Korean patients with AF undergoing PCI have shifted toward less DAPT and more TAT. NOACs are increasingly used when OAC is prescribed; however, DAPT was still the most common regimen during the periprocedural period.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download