INTRODUCTION
Long-term exercise training may result in structural and functional changes of the heart to adapt the increased hemodynamic demands, referred to athlete's heart syndrome or exercise induced cardiac remodeling (EICR).
1) Various factors including sports type, frequency and intensity of athletic training, gender, and age are known to be associated with these structural and functional changes in athletes.
2)
Traditionally, left ventricle (LV) is the major concern for the athletic changes of cardiac structure and function, and LV hypertrophy or chamber enlargement has been the most commonly described cardiac abnormality. Recent studies, however, have demonstrated that cardiac structural and functional changes can be developed not only in the LV, but also in the left atrium (LA) and right ventricle (RV).
3)4) Because the enlargement of the LA or RV or LV hypertrophy is associated with cardiovascular disease (CVD) or adverse cardiovascular (CV) outcomes, it is very important for physicians to differentiate these physiologic changes of the heart among highly trained athletes from pathologic conditions, especially in sports counseling.
5)
Over past 2 decades, the importance of CVD in women and gender difference in CVD and CV outcomes have been an important research theme, and, nowadays, gender difference has been demonstrated or established in various CVD.
6)7) Although athlete heart syndrome is now a well-established phenomenon in highly trained athletes, gender difference in athletic changes of the cardiac structure and function has been poorly studied and only a few studies with limited number of study subjects have been conducted till now.
8)9) Colombo and Finocchiaro
10) recently, reported gender difference in LV geometric changes in a large group of athletes, but gender difference in cardiac chambers other than LV such as LA or RV has not been evaluated in a large group of athletes. Therefore, we investigated gender difference in EICR not only for LV, but also for LA or RV, and we also investigated sex-specific predictors of cardiac remodeling in a large group of highly trained university athletes.
Go to :

METHODS
Check-up your heart program
To screen structural and functional changes or abnormalities of university athletes, the International University Sports Federation (FISU) planned the Check-up Your Heart Program as a legacy project of the 2015 Gwangju Summer Universiade. In collaboration with FISU and the organizing committee of the 2015 Gwangju Summer Universiade, the Check-up Your Heart Program was operated in the Athlete Village on 1–14 July 2015, during the Universiade.
In addition, personnel for this program were as follows: 4 cardiologists (1 electrophysiologist and 3 echocardiologists), 3 cardiac sonographers who have a license of Registered Diagnostic Cardiac Sonographer of the USA, 1 medical technologist, nine volunteers, and 2 translators. Demographic information was obtained using the athlete-reported questionnaire. Blood pressure, heart rate (HR), and anthropometric data including height, weight, and wingspan were collected. Body composition including lean body mass, body fat mass, and muscle mass were measured by using InBody 230 Body Composition Analyzer (InBody Co., Cerritos, CA, USA).
Study population
A total of 1,185 university athletes from 140 countries gave informed consent and completed the CV screening program during the 2015 Gwangju Summer Universiade.
According to sex, athletes were divided into 2 groups; female athletes (n=497, 22.0±2.3 years) vs. male athletes (n=688, 22.6±2.4 years). Remodeling of the LV, LA, RV, and any cardiac chamber were compared. Demographic, anthropometric, and echocardiographic findings were also compared between the groups for LV, LA, RV, and any cardiac chamber remodeling.
Definitions of the study
LV remodeling was defined as the presence of any abnormality in LV geometry on echocardiography. LV geometry was classified into 4 categories according to the current guideline for chamber quantification: normal, concentric remodeling, concentric hypertrophy, and eccentric hypertrophy.
11)
LA remodeling was defined as the increased LA volume index (LAVI) more than 42 mL/m
2 in accordance with our recently published study.
12)
RV remodeling was defined as an indexed RV outflow tract (RVOT) diameter >19 mm/m
2 in parasternal long axis image or >21 mm/m
2 in short axis image on echocardiography.
13)
Any remodeling was defined as the presence of any remodeling in LV, LA, or RV.
Sports types were classified into groups according to the intensity of static (I: low; II: moderate; III: high) and dynamic components (A: low; B: moderate; C: high); lowest (IA), including golf, bowling, cricket, curling, golf; low moderate (IIA, including diving, equestrian, motorcycling, IB, including baseball, fencing, table tennis, volleyball); moderate (IIIA, including gymnastics, sailing, water skiing, weight lifting, IIB including sprint running, surfing, synchronized swimming, jumping, IC, including badminton, field hockey, long distance running); high moderate (IIIB, including body building, wrestling, IIC, including basketball, swimming, team handball, middle distance running) and highest CV demands (IIIC, including boxing, canoeing, cycling, rowing, triathlon).
14)
Echocardiographic examination
Comprehensive 2-dimensional (2D) and Doppler echocardiographic examinations were performed in accordance with the recommendation of the current guideline.
15) Echocardiographic images from various echocardiographic windows were obtained by using a digital ultrasonographic equipment system (Vivid 7; GE Vingmed Ultrasound, Horten, Norway). All of the data were analyzed by using the computerized offline software package (EchoPAC PC 6.0.0; GE Vingmed Ultrasound).
The 2D measurements of the left heart included LV end-diastolic dimension (LVEDD), LV end-systolic dimension, thickness of the LV interventricular septum (IVS) and posterior wall, LV end-diastolic and end-systolic volume, LV ejection fraction (LVEF), LV mass. Relative wall thickness (RWT) was defined as the ratio of the sum of the IVS and posterior wall thickness in end-diastole to the LVEDD, and RWT >0.42 was considered abnormal in both sexes. LV mass index >115 g/m2 in men and >95 g/m2 in women was considered abnormal. Based on RWT and LV mass, LV geometry was classified into 4 categories as previous described.
LA measurements were performed at left ventricular end-systole, in atrial maximal size. The LA area was measured in a 4-chamber view. LA volume was calculated by the Simpson’s method and then indexed to the body surface area (BSA) as a LAVI. During LA size assessment, the LA appendage and pulmonary veins were excluded, and mitral valve annulus plane was used as the inferior border.
The 2D measurements of the right heart include RV basal and longitudinal diameter in the apical view and RVOT diameter in the parasternal short-axis view. RV wall thickness, tricuspid annular plane systolic excursion (TAPSE), and RV systolic pressure were obtained. The diameter of the inferior vena cava was also included in RV measurements.
Global longitudinal strain (GLS) of the LV was measured by automate function imaging (AFI). After selecting the optimal 2D image, the timing of aortic valve closure was derived from the pulse wave Doppler of the aortic valve, and the 3-point click method in 3 apical planes (apical 4-chamber, 2-chamber, and long axis view) was used. AFI non-invasively tracked and analyzed GLS based on the 2D speckle tracking method and displayed the combined results of GLS of the 3 planes in a single bull's eye summary. Inter-and intra-observer variability of GLS was 0.957 and 0.962 respectively. The endocardial border of the LA was traced on the best echocardiographic image at the end diastolic phase. After the adjustment of region of interest to the thickness of the LA, the software tracked the change of speckles on subsequent frames automatically with R–R gating as the zero-reference point. Because the tracing was verified in real-time, it could be corrected by manually adjusting the contour to obtain acceptable tracking result. The average LA reservoir strain (peak atrial longitudinal strain) and contractile strain (pump function) were measured from apical 4-chamber view and apical 2 chamber view. We used average value from 2 apical views.
16) Intra-observer variability of LA reservoir strain and contractile strain were 0.957 and 0.959, respectively. Inter-observer variability of LA reservoir strain and contractile strain were 0.938 and 0.950, respectively.
Statistical analyses
The Statistical Package for Social Sciences version 18.0 for Windows (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis. Data are presented as percentages or mean±standard deviation. The differences in the categorical variables were evaluated by using the χ2 test, and the continuous variables were compared by using the independent t-test.
To identify the independent predictor of cardiac remodeling, multivariate logistic regression analysis was applied to the significant variables in the univariate analysis.
A p value of <0.05 was considered as statistically significant.
Ethics statement
The study protocol was approved by the Institutional Review Board (IRB No. CNUH-2015-135).
Go to :

RESULTS
Baseline characteristics
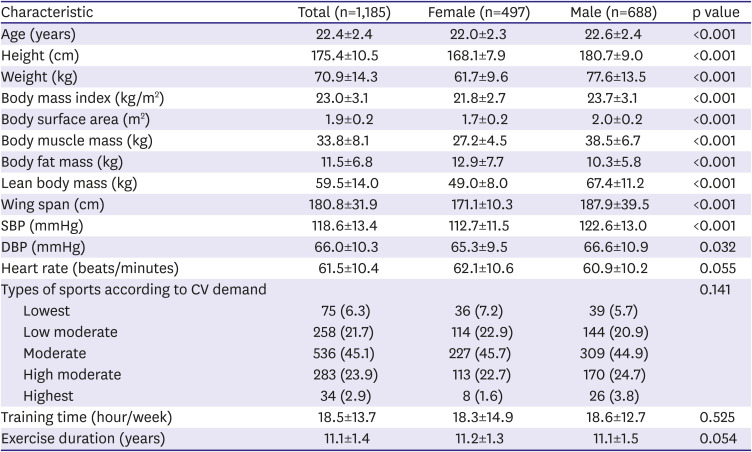
Baseline characteristics between the groups are summarized in
Table 1.
Table 1
Baseline characteristics
|
Characteristic |
Total (n=1,185) |
Female (n=497) |
Male (n=688) |
p value |
|
Age (years) |
22.4±2.4 |
22.0±2.3 |
22.6±2.4 |
<0.001 |
|
Height (cm) |
175.4±10.5 |
168.1±7.9 |
180.7±9.0 |
<0.001 |
|
Weight (kg) |
70.9±14.3 |
61.7±9.6 |
77.6±13.5 |
<0.001 |
|
Body mass index (kg/m2) |
23.0±3.1 |
21.8±2.7 |
23.7±3.1 |
<0.001 |
|
Body surface area (m2) |
1.9±0.2 |
1.7±0.2 |
2.0±0.2 |
<0.001 |
|
Body muscle mass (kg) |
33.8±8.1 |
27.2±4.5 |
38.5±6.7 |
<0.001 |
|
Body fat mass (kg) |
11.5±6.8 |
12.9±7.7 |
10.3±5.8 |
<0.001 |
|
Lean body mass (kg) |
59.5±14.0 |
49.0±8.0 |
67.4±11.2 |
<0.001 |
|
Wing span (cm) |
180.8±31.9 |
171.1±10.3 |
187.9±39.5 |
<0.001 |
|
SBP (mmHg) |
118.6±13.4 |
112.7±11.5 |
122.6±13.0 |
<0.001 |
|
DBP (mmHg) |
66.0±10.3 |
65.3±9.5 |
66.6±10.9 |
0.032 |
|
Heart rate (beats/minutes) |
61.5±10.4 |
62.1±10.6 |
60.9±10.2 |
0.055 |
|
Types of sports according to CV demand |
|
|
|
0.141 |
|
Lowest |
75 (6.3) |
36 (7.2) |
39 (5.7) |
|
Low moderate |
258 (21.7) |
114 (22.9) |
144 (20.9) |
|
Moderate |
536 (45.1) |
227 (45.7) |
309 (44.9) |
|
High moderate |
283 (23.9) |
113 (22.7) |
170 (24.7) |
|
Highest |
34 (2.9) |
8 (1.6) |
26 (3.8) |
|
Training time (hour/week) |
18.5±13.7 |
18.3±14.9 |
18.6±12.7 |
0.525 |
|
Exercise duration (years) |
11.1±1.4 |
11.2±1.3 |
11.1±1.5 |
0.054 |

Co-morbid diseases were not identified in all participants. Male athletes were more commonly enrolled (58.1%). Mean age was 22 years in both sexes. Body mass index, BSA, body muscle mass, lean body mass, and wing span were larger in males, whereas body fat mass was larger in females. Systolic and diastolic blood pressures were higher in males than in females. Sports type, training time, and exercise duration were not different in both groups.
Echocardiographic findings
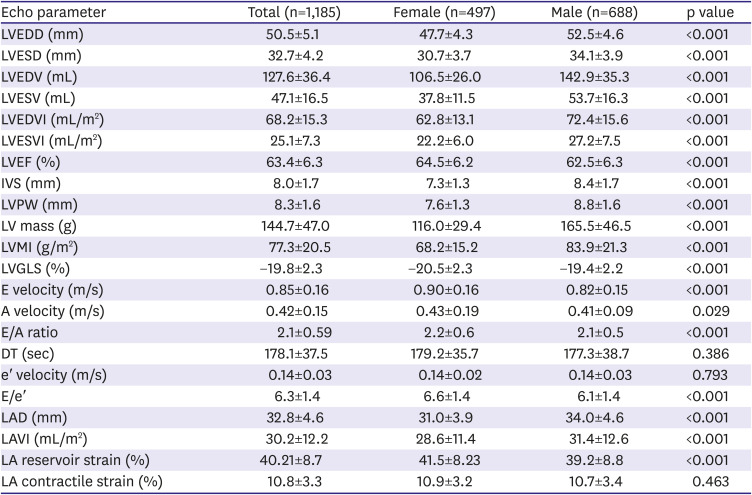
Echocardiographic findings of the left heart in university athletes are shown in
Table 2.
Table 2
Echocardiographic findings of the left heart
|
Echo parameter |
Total (n=1,185) |
Female (n=497) |
Male (n=688) |
p value |
|
LVEDD (mm) |
50.5±5.1 |
47.7±4.3 |
52.5±4.6 |
<0.001 |
|
LVESD (mm) |
32.7±4.2 |
30.7±3.7 |
34.1±3.9 |
<0.001 |
|
LVEDV (mL) |
127.6±36.4 |
106.5±26.0 |
142.9±35.3 |
<0.001 |
|
LVESV (mL) |
47.1±16.5 |
37.8±11.5 |
53.7±16.3 |
<0.001 |
|
LVEDVI (mL/m2) |
68.2±15.3 |
62.8±13.1 |
72.4±15.6 |
<0.001 |
|
LVESVI (mL/m2) |
25.1±7.3 |
22.2±6.0 |
27.2±7.5 |
<0.001 |
|
LVEF (%) |
63.4±6.3 |
64.5±6.2 |
62.5±6.3 |
<0.001 |
|
IVS (mm) |
8.0±1.7 |
7.3±1.3 |
8.4±1.7 |
<0.001 |
|
LVPW (mm) |
8.3±1.6 |
7.6±1.3 |
8.8±1.6 |
<0.001 |
|
LV mass (g) |
144.7±47.0 |
116.0±29.4 |
165.5±46.5 |
<0.001 |
|
LVMI (g/m2) |
77.3±20.5 |
68.2±15.2 |
83.9±21.3 |
<0.001 |
|
LVGLS (%) |
−19.8±2.3 |
−20.5±2.3 |
−19.4±2.2 |
<0.001 |
|
E velocity (m/s) |
0.85±0.16 |
0.90±0.16 |
0.82±0.15 |
<0.001 |
|
A velocity (m/s) |
0.42±0.15 |
0.43±0.19 |
0.41±0.09 |
0.029 |
|
E/A ratio |
2.1±0.59 |
2.2±0.6 |
2.1±0.5 |
<0.001 |
|
DT (sec) |
178.1±37.5 |
179.2±35.7 |
177.3±38.7 |
0.386 |
|
e′ velocity (m/s) |
0.14±0.03 |
0.14±0.02 |
0.14±0.03 |
0.793 |
|
E/e′ |
6.3±1.4 |
6.6±1.4 |
6.1±1.4 |
<0.001 |
|
LAD (mm) |
32.8±4.6 |
31.0±3.9 |
34.0±4.6 |
<0.001 |
|
LAVI (mL/m2) |
30.2±12.2 |
28.6±11.4 |
31.4±12.6 |
<0.001 |
|
LA reservoir strain (%) |
40.21±8.7 |
41.5±8.23 |
39.2±8.8 |
<0.001 |
|
LA contractile strain (%) |
10.8±3.3 |
10.9±3.2 |
10.7±3.4 |
0.463 |

LV dimensions and volumes were significantly smaller and LV wall thickness and muscle mass were significantly lower in female athletes than in male athletes, whereas LVEF and GLS were significantly higher in female athletes than in male athletes. LV volumes and mass were significantly lower in female than in male athletes even after indexing to BSA. E velocity of mitral inflow and E/e′ were significantly higher in female athletes than in male athletes.
LA size and volume were significantly smaller in female athletes than in male athletes, whereas LA reservoir strain was significantly higher in female athletes than in male athletes.
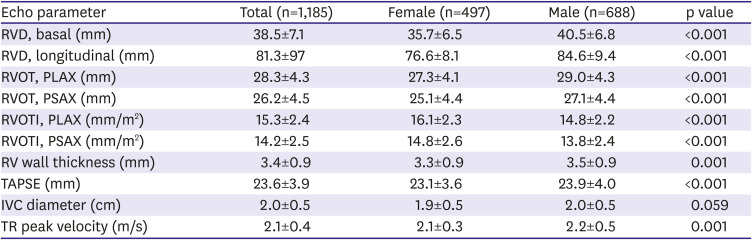
Echocardiographic findings of the right heart are summarized in
Table 3.
Table 3
Echocardiographic findings of the right heart
|
Echo parameter |
Total (n=1,185) |
Female (n=497) |
Male (n=688) |
p value |
|
RVD, basal (mm) |
38.5±7.1 |
35.7±6.5 |
40.5±6.8 |
<0.001 |
|
RVD, longitudinal (mm) |
81.3±97 |
76.6±8.1 |
84.6±9.4 |
<0.001 |
|
RVOT, PLAX (mm) |
28.3±4.3 |
27.3±4.1 |
29.0±4.3 |
<0.001 |
|
RVOT, PSAX (mm) |
26.2±4.5 |
25.1±4.4 |
27.1±4.4 |
<0.001 |
|
RVOTI, PLAX (mm/m2) |
15.3±2.4 |
16.1±2.3 |
14.8±2.2 |
<0.001 |
|
RVOTI, PSAX (mm/m2) |
14.2±2.5 |
14.8±2.6 |
13.8±2.4 |
<0.001 |
|
RV wall thickness (mm) |
3.4±0.9 |
3.3±0.9 |
3.5±0.9 |
0.001 |
|
TAPSE (mm) |
23.6±3.9 |
23.1±3.6 |
23.9±4.0 |
<0.001 |
|
IVC diameter (cm) |
2.0±0.5 |
1.9±0.5 |
2.0±0.5 |
0.059 |
|
TR peak velocity (m/s) |
2.1±0.4 |
2.1±0.3 |
2.2±0.5 |
0.001 |

RV sizes in various imaging planes were significantly smaller in female than in male athletes, but the indexed RVOT dimensions to BSA were significantly larger in female than in male athletes. TAPSE and TR peak velocity were significantly lower and RV wall thickness was significantly thinner in female than in male athletes.
Gender and exercise induced cardiac remodeling
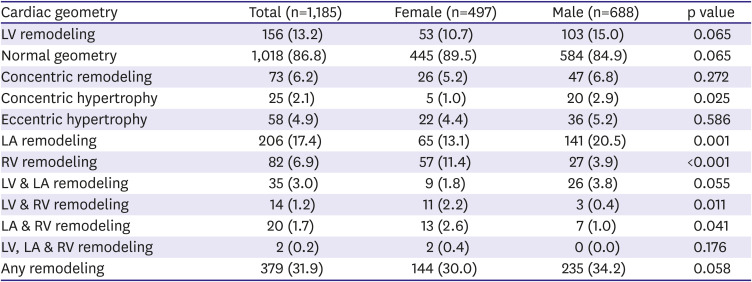
Patterns of EICR in university athletes are shown in
Table 4.
Table 4
Patterns of exercise induced cardiac remodeling
|
Cardiac geometry |
Total (n=1,185) |
Female (n=497) |
Male (n=688) |
p value |
|
LV remodeling |
156 (13.2) |
53 (10.7) |
103 (15.0) |
0.065 |
|
Normal geometry |
1,018 (86.8) |
445 (89.5) |
584 (84.9) |
0.065 |
|
Concentric remodeling |
73 (6.2) |
26 (5.2) |
47 (6.8) |
0.272 |
|
Concentric hypertrophy |
25 (2.1) |
5 (1.0) |
20 (2.9) |
0.025 |
|
Eccentric hypertrophy |
58 (4.9) |
22 (4.4) |
36 (5.2) |
0.586 |
|
LA remodeling |
206 (17.4) |
65 (13.1) |
141 (20.5) |
0.001 |
|
RV remodeling |
82 (6.9) |
57 (11.4) |
27 (3.9) |
<0.001 |
|
LV & LA remodeling |
35 (3.0) |
9 (1.8) |
26 (3.8) |
0.055 |
|
LV & RV remodeling |
14 (1.2) |
11 (2.2) |
3 (0.4) |
0.011 |
|
LA & RV remodeling |
20 (1.7) |
13 (2.6) |
7 (1.0) |
0.041 |
|
LV, LA & RV remodeling |
2 (0.2) |
2 (0.4) |
0 (0.0) |
0.176 |
|
Any remodeling |
379 (31.9) |
144 (30.0) |
235 (34.2) |
0.058 |

Any remodeling in cardiac chambers was relatively common in both female and male athletes. About a third of university athletes experienced any remodeling in cardiac chambers, and any remodeling was more prevalent in male athletes than in female athletes.
LV and LA remodeling were more common in male than female athletes, whereas RV remodeling was more common in female than in male athletes. Concentric hypertrophy was significantly frequent in male than in female athletes, but other sub-types of LV remodeling were not different between the groups.
Interestingly, the simultaneous development of multi-chamber remodeling was not common in both sexes. In other words, regardless of gender, the development of LV, LA, and RV remodeling were not overlapped in most of athletes, suggesting the different mechanism of EICR according to cardiac chambers in both sexes (
Figure 1).
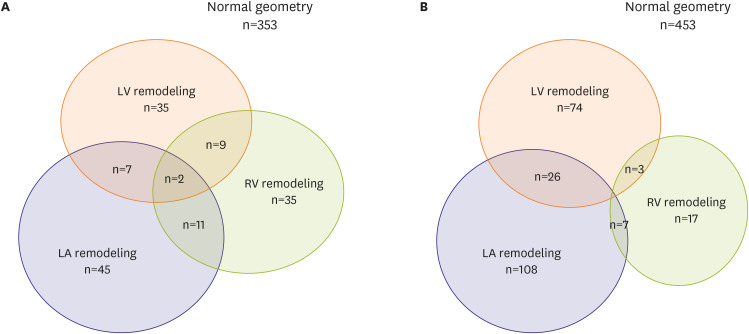 | Figure 1
Gender-specific exercise induced cardiac remodeling in university athletes (A) female athletes, (B) male athletes.
LA = left atrium; LV = left ventricle; RV = right ventricle.

|
Sports type and exercise induced cardiac remodeling
As the increase of CV demands according to sports type, regardless of gender, the incidence of EICR including LV, LA, RV, and any remodeling were significantly increased (
Figure 2).
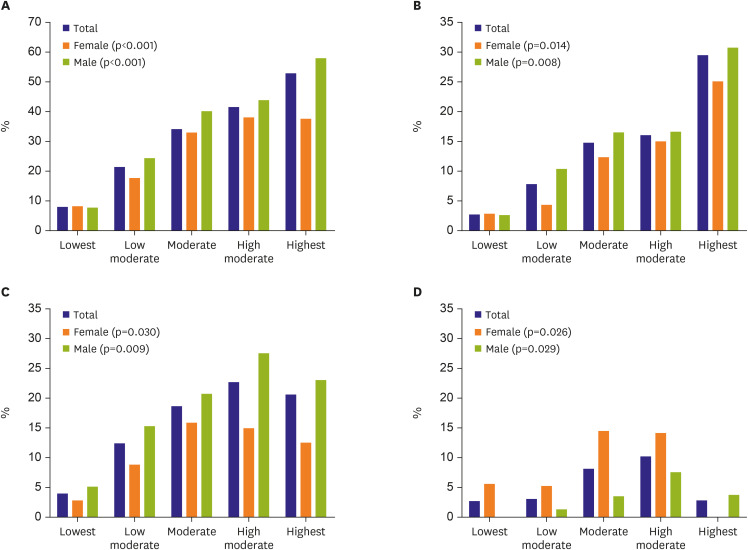 | Figure 2
Gender-specific exercise induced cardiac remodeling according to sports type classified by cardiovascular demand. (A) Any remodeling, (B) LV remodeling, (C) LA remodeling, (D) RV remodeling.
LA = left atrium; LV = left ventricle; RV = right ventricle.

|
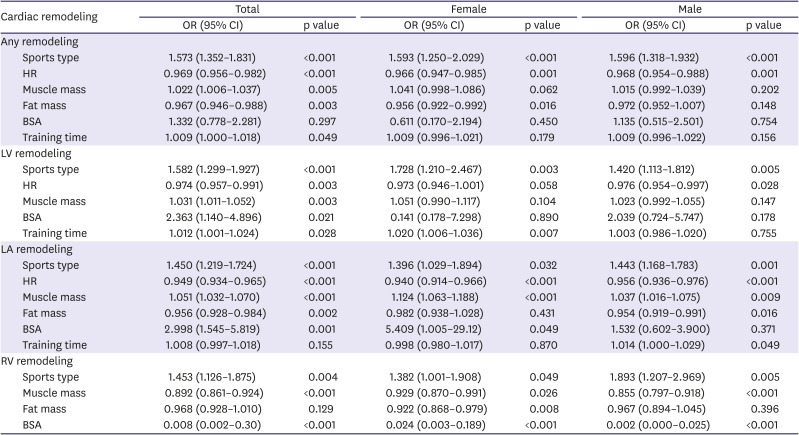
Independent predictors of exercise induced cardiac remodeling
In univariate analysis, height, BSA, systolic blood pressure, HR, sports type according to CV demands, muscle mass, fat mass, and training time, LVEF, LV GLS were significantly associated with EICR.
To identify the independent predictors of EICR, multivariate regression analysis including significant variables in univariate analysis was performed. Predictors of EICR are summarized in
Table 5.
Table 5
Multivariate analysis for exercise induced cardiac remodeling
|
Cardiac remodeling |
Total |
Female |
Male |
|
OR (95% CI) |
p value |
OR (95% CI) |
p value |
OR (95% CI) |
p value |
|
Any remodeling |
|
|
|
|
|
|
|
Sports type |
1.573 (1.352–1.831) |
<0.001 |
1.593 (1.250–2.029) |
<0.001 |
1.596 (1.318–1.932) |
<0.001 |
|
HR |
0.969 (0.956–0.982) |
<0.001 |
0.966 (0.947–0.985) |
0.001 |
0.968 (0.954–0.988) |
0.001 |
|
Muscle mass |
1.022 (1.006–1.037) |
0.005 |
1.041 (0.998–1.086) |
0.062 |
1.015 (0.992–1.039) |
0.202 |
|
Fat mass |
0.967 (0.946–0.988) |
0.003 |
0.956 (0.922–0.992) |
0.016 |
0.972 (0.952–1.007) |
0.148 |
|
BSA |
1.332 (0.778–2.281) |
0.297 |
0.611 (0.170–2.194) |
0.450 |
1.135 (0.515–2.501) |
0.754 |
|
Training time |
1.009 (1.000–1.018) |
0.049 |
1.009 (0.996–1.021) |
0.179 |
1.009 (0.996–1.022) |
0.156 |
|
LV remodeling |
|
|
|
|
|
|
|
Sports type |
1.582 (1.299–1.927) |
<0.001 |
1.728 (1.210–2.467) |
0.003 |
1.420 (1.113–1.812) |
0.005 |
|
HR |
0.974 (0.957–0.991) |
0.003 |
0.973 (0.946–1.001) |
0.058 |
0.976 (0.954–0.997) |
0.028 |
|
Muscle mass |
1.031 (1.011–1.052) |
0.003 |
1.051 (0.990–1.117) |
0.104 |
1.023 (0.992–1.055) |
0.147 |
|
BSA |
2.363 (1.140–4.896) |
0.021 |
0.141 (0.178–7.298) |
0.890 |
2.039 (0.724–5.747) |
0.178 |
|
Training time |
1.012 (1.001–1.024) |
0.028 |
1.020 (1.006–1.036) |
0.007 |
1.003 (0.986–1.020) |
0.755 |
|
LA remodeling |
|
|
|
|
|
|
|
Sports type |
1.450 (1.219–1.724) |
<0.001 |
1.396 (1.029–1.894) |
0.032 |
1.443 (1.168–1.783) |
0.001 |
|
HR |
0.949 (0.934–0.965) |
<0.001 |
0.940 (0.914–0.966) |
<0.001 |
0.956 (0.936–0.976) |
<0.001 |
|
Muscle mass |
1.051 (1.032–1.070) |
<0.001 |
1.124 (1.063–1.188) |
<0.001 |
1.037 (1.016–1.075) |
0.009 |
|
Fat mass |
0.956 (0.928–0.984) |
0.002 |
0.982 (0.938–1.028) |
0.431 |
0.954 (0.919–0.991) |
0.016 |
|
BSA |
2.998 (1.545–5.819) |
0.001 |
5.409 (1.005–29.12) |
0.049 |
1.532 (0.602–3.900) |
0.371 |
|
Training time |
1.008 (0.997–1.018) |
0.155 |
0.998 (0.980–1.017) |
0.870 |
1.014 (1.000–1.029) |
0.049 |
|
RV remodeling |
|
|
|
|
|
|
|
Sports type |
1.453 (1.126–1.875) |
0.004 |
1.382 (1.001–1.908) |
0.049 |
1.893 (1.207–2.969) |
0.005 |
|
Muscle mass |
0.892 (0.861–0.924) |
<0.001 |
0.929 (0.870–0.991) |
0.026 |
0.855 (0.797–0.918) |
<0.001 |
|
Fat mass |
0.968 (0.928–1.010) |
0.129 |
0.922 (0.868–0.979) |
0.008 |
0.967 (0.894–1.045) |
0.396 |
|
BSA |
0.008 (0.002–0.30) |
<0.001 |
0.024 (0.003–0.189) |
<0.001 |
0.002 (0.000–0.025) |
<0.001 |

Overall, sports type according to CV demands, HR, muscle mass, fat mass, and training time were independent predictors of any remodeling. In female athletes, sports type, HR, and fat mass were independent predictors of any remodeling, but muscle mass and training time were not a predictor of any remodeling. In male, sports type and HR were independent predictors of any remodeling, but muscle mass, fat mass and training time were not independent predictors of any remodeling.
Overall, sports type, HR, muscle mass, BSA, and training time were independent predictors of LV remodeling. In female, sports type and training time were independent predictors of LV remodeling, but HR, muscle mass and BSA were not independent predictors. In male, sports type and HR were independent predictors of LV remodeling, but muscle mass, BSA, and training time were not predictors.
Overall, sports type, HR, muscle mass, fat mass, and BSA were independent predictors of LA remodeling. In female, sports type, HR, muscle mass, and BSA were independent predictors of LA remodeling, but fat mass and BSA were not predictors. In male, sports type, HR, muscle mass, and fat mass were independent predictors of LA remodeling, but BSA was not predictors. However, training time was an independent predictor of RV remodeling in male athletes, but not in female athletes.
Overall, sports type, muscle mass and BSA were independent predictors of RV remodeling in both sexes. However, fat mass was an independent predictor of RV remodeling in female athletes, but not in male athletes.
Go to :

DISCUSSION
The authors investigated gender difference in EICR and gender-specific predictors of EICR in a large group of highly trained university athletes. Also, the present study demonstrated several important findings regarding athlete heart syndrome in university athletes, which would be helpful for gender-specific sports counseling.
First, EICR developed not only in LV, but also in LA or RV in highly trained university athletes, and, regardless of gender, remodeling in at least one cardiac chamber is relatively common and developed in about a third of university athletes. Second, LV or LA remodeling was significantly more common in male than in female athletes, whereas RV remodeling was more common in female than in male athletes demonstrating gender difference of EICR. Third, the development of LV, LA, and RV remodeling was not overlapped in most of the athletes irrespective of gender, suggesting the different mechanism of EICR according to cardiac chambers. Fourth, various predictors including sports type, HR, muscle mass, fat mass, BSA, and training time were differently involved in EICR, and gender differences of these predictors of EICR were also demonstrated through this study.
The prevalence of LV remodeling in athletes quite varies among studies according to the applied methods of LV measurements or athletic population. To evaluate LV remodeling, the authors adopted the LV geometry pattern of the current guideline,
11) as used in the study of Colombo and Finocchiaro.
10) Although abnormal LV geometry was noted in 30% of athletes in the study of Finocchiaro and Sharma,
17) LV remodeling was noted in only 14.1% of university athletes in this study. Different types of sports in university athletes would be a possible explanation of this difference in the prevalence of LV remodeling. Therefore, further well-designed studies including a large number of athletes with the same types of sports will be needed to elucidate the true prevalence of EICR.
LA enlargement is also an important part of EICR. In the meta-analysis of Iskandar et al.,
3) LA diameter was 4.1 mm greater and LAVI was 7.0 mL/m
2 greater in athletes than in controls. In the present study, the authors adopted LAVI >42 mL/m
2 as a LA remodeling instead of LAVI >34 mL/m
2, which is the conventional cut-off value of LA enlargement for this and other reasons,
12) and the prevalence of EICR was 17.4% in university athletes. Nevertheless, LA was the most commonly affected cardiac chamber than other cardiac chambers in terms of EICR, and the prevalence of LA remodeling would certainly be higher if we use LAVI >34 mL/m
2 as a definition. In spite of the relatively high prevalence of LA remodeling, the authors cannot explain the clinical significance of LA remodeling in athletes yet. Therefore, the clinical significance of LA remodeling in athletes should be clarified through a future larger longitudinal cohort study.
In our previous report,
18) abnormally enlarged RVOT diameter was noted in 21.3% of athletes. Because body size and habitus of athletes are usually different from those of the general population, we adopted an indexed RVOT diameter to BSA >19 mm/m
2 in parasternal long-axis image or >21 mm/m
2 in short-axis image as a definition of RV remodeling in the present study as used in the recent recommendation or study.
13)19) By this definition, RV remodeling was noted in 7.1% of university athletes, and it was quite similar to the prevalence of RVOT enlargement in the study of D'Ascenzi et al.,
20) which used arrhythmogenic RV cardiomyopathy criteria. Because the prevalence of RV remodeling may vary according to the used parameters for definition, a standardized definition for RV remodeling with clinical significance should be established for future research.
EICR in any of cardiac chambers was common in the present study and developed in about a third (32.7%) of highly trained university athletes. And the prevalence of EICR was more than 2 times higher in the left-side than in the right-side of the heart. Because an indexed RVOT dimension, among various parameters, was the only parameter for the definition of RV remodeling in this study, the prevalence of RV remodeling might be underestimated in the present study. Interestingly, the development of LV, LA, and RV remodeling were not overlapped in most of athletes irrespective of gender, suggesting the different mechanism of EICR according to cardiac chambers.
EICR can develop in both sexes, but it is more pronounced among male athletes, especially in the aspect of the left side heart. Female was found to have 23% less LV wall thickness and 11% smaller LV size as compared to those of male athletes with similar age and training regimen.
21) Females, as compared to males, are small, have lower lean body mass, and different hormonal factors that have a significant impact on cardiac dimensions.
22) The present study also demonstrated a gender difference in EICR that LV remodeling is more prominent in male than female university athletes. LA remodeling was also more common in male than female athletes in the present study. In contrary to EICR of left-sided heart, however, RV remodeling was more common in females than in male athletes suggesting gender difference of EICR according to the affected cardiac chambers. LV and LA volumes were larger in male than in female athletes whether they are indexed to BSA or not. However, the indexed RVOT dimension to BSA was significantly larger in female than male athletes, even though the absolute value of the RVOT dimension was significantly smaller in female than in male athletes. Zaidi et al.
22) reported similar findings that absolute RV dimensions are larger in male athletes than in females, but RV size indexed for BSA is higher in females than in male athletes.
17)23) Based on these observations, gender differences in RV remodeling should be considered in evaluating the right heart of athletes.
The exact mechanism for higher RV remodeling in female than in male university athletes is unclear and needed to be clarified. The law of Laplace, sex, and body size may be involved in RV remodeling in athletes.
24) Data from an animal study demonstrated sex-specific regulation of physiological hypertrophy in response to exercise. Females showed an increased hypertrophic response to training load compared to males. It was hypothesized that sex hormone might be a modulator of the development and progression of cardiac adaptation.
25) Other animal studies have shown increased plasma free fatty acid (FFA) levels and augmented lipolysis in the female after training when compared to males, indicative of preferential fatty acid utilization.
26) Female has higher rates of lipid oxidation compared with male resulting from enhanced adrenergic lipolysis and increased plasma FFA levels. Exercise-induced increases in plasma FFA level are closely linked to a decrease in cardiac glucose uptake in the female. With the use of cardiac PET analysis, increased post-training plasma FFA levels in women compared with men suggest the predominance of lipids as the “female” cardiac fuel during exercise.
27)28) Sympathetic adrenergic response during exercise affected differently in female athletes.
29) Body composition such as fat mass, volume sensitivity, and metabolic preference may affect the difference of EICR according to sex.
This study has several potential limitations. First, because the present study is a cross-sectional study, the clinical significance of gender difference in EICR and its impacts on clinical outcomes cannot be evaluated. A longitudinal follow-up study would be warranted to elucidate the clinical significance and gender difference of EICR. Second, because the present study was operated during the limited period of Universiade and Check-up Your Heart Program was a voluntary program, there might be a chance of selection bias, especially in regard to gender, race, country, and sports disciplines. Therefore, the results of this study should be cautiously interpreted and applied for sports counseling. Third, as discussed in the discussion, the prevalence of RV remodeling might be underestimated in this study because the authors adopted relatively strict criteria as a definition of RV remodeling. Standard criteria for RV remodeling should be developed for future research. Fourth, unfortunately, the data about RA size and volume was not available. If we had the RA remodeling data, it would have been able to show the whole cardiac chamber remodeling.
In conclusion, the present study demonstrated that EICR was common in both sexes and was independently developed among cardiac chambers in highly trained university athletes. LV and LA remodeling were significantly common in males, whereas RV remodeling was significantly common in females demonstrating gender difference in cardiac remodeling. Gender difference in the predictors of EICR was also demonstrated. Therefore, gender-specific EICR and predictors of EICR should be considered in evaluating the cardiac structure and function of athletes.
Go to :










 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download