1. Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev. 2011; 91:733–794. PMID:
21527736.

2. DeFronzo RA, Reeves WB, Awad AS. Pathophysiology of diabetic kidney disease: impact of SGLT2
inhibitors. Nat Rev Nephrol. 2021.
3. Zelniker TA, Braunwald E. Mechanisms of cardiorenal effects of sodium-glucose cotransporter 2
inhibitors: JACC state-of-the-art review. J Am Coll Cardiol. 2020; 75:422–434. PMID:
32000955.
4. Alicic RZ, Neumiller JJ, Johnson EJ, Dieter B, Tuttle KR. Sodium-glucose cotransporter 2 inhibition and diabetic kidney
disease. Diabetes. 2019; 68:248–257. PMID:
30665953.
5. Gilbert RE, Krum H. Heart failure in diabetes: effects of anti-hyperglycaemic drug
therapy. Lancet. 2015; 385:2107–2117. PMID:
26009231.
6. Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection
fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020; 396:819–829. PMID:
32877652.
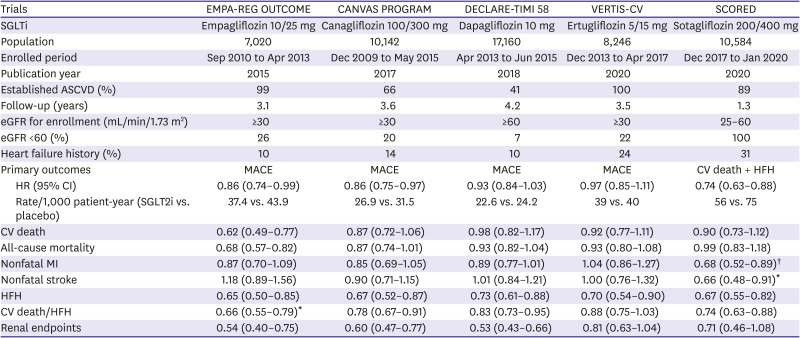
7. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2
diabetes. N Engl J Med. 2015; 373:2117–2128. PMID:
26378978.
8. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2
diabetes. N Engl J Med. 2019; 380:347–357. PMID:
30415602.
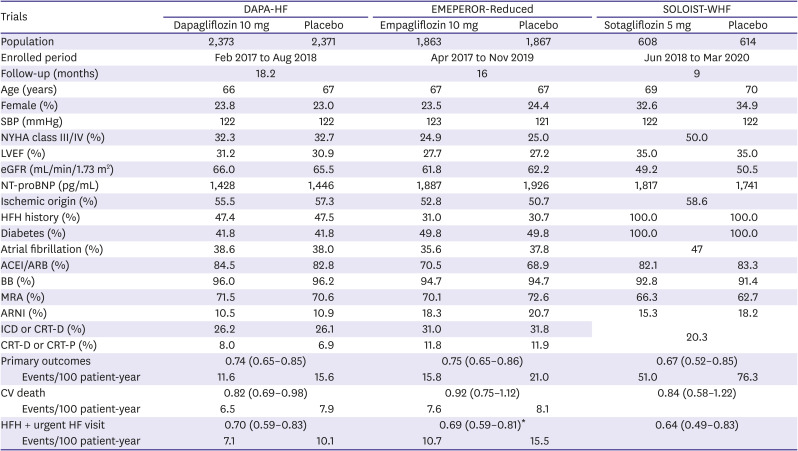
9. McMurray JJ, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection
fraction. N Engl J Med. 2019; 381:1995–2008. PMID:
31535829.
10. Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart
failure. N Engl J Med. 2020; 383:1413–1424. PMID:
32865377.
11. Pellicori P, Ofstad AP, Fitchett D, et al. Early benefits of empagliflozin in patients with or without heart failure:
findings from EMPA-REG OUTCOME. ESC Heart Fail. 2020; 7:3401–3407.
12. Verma S, McMurray JJ. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a
state-of-the-art review. Diabetologia. 2018; 61:2108–2117. PMID:
30132036.
13. Neal B, Perkovic V, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2
diabetes. N Engl J Med. 2017; 377:2099. PMID:
29166232.
14. Cannon CP, Pratley R, Dagogo-Jack S, et al. Cardiovascular outcomes with ertugliflozin in type 2
diabetes. N Engl J Med. 2020; 383:1425–1435. PMID:
32966714.
15. Bhatt DL, Szarek M, Pitt B, et al. Sotagliflozin in patients with diabetes and chronic kidney
disease. N Engl J Med. 2021; 384:129–139. PMID:
33200891.
16. Zannad F, Cowie MR. VERTIS-CV: more evidence that sodium glucose cotransporter 2 inhibition
brings rapid and sustained heart failure benefit. Circulation. 2020; 142:2216–2218. PMID:
33284652.
17. Kramer CK, Ye C, Campbell S, Retnakaran R. Comparison of new glucose-lowering drugs on risk of heart failure in type 2
diabetes: a network meta-analysis. JACC Heart Fail. 2018; 6:823–830. PMID:
30196071.
18. Fitchett D, Zinman B, Wanner C, et al. Heart failure outcomes with empagliflozin in patients with type 2 diabetes
at high cardiovascular risk: results of the EMPA-REG OUTCOME
®
trial. Eur Heart J. 2016; 37:1526–1534. PMID:
26819227.
19. Grant PJ, Cosentino F. The 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular
diseases developed in collaboration with the EASD: New features and the ‘Ten
Commandments’ of the 2019 guidelines are discussed by Professor Peter J. Grant
and Professor Francesco Cosentino, the Task Force chairmen. Eur Heart J. 2019; 40:3215–3217. PMID:
31608951.
20. Kato ET, Silverman MG, Mosenzon O, et al. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes
mellitus. Circulation. 2019; 139:2528–2536. PMID:
30882238.
21. Docherty KF, McMurray JJ. SOLOIST-WHF and updated meta-analysis: sodium-glucose co-transporter 2
inhibitors should be initiated in patients hospitalized with worsening heart
failure. Eur J Heart Fail. 2021; 23:27–30. PMID:
33283384.
22. Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in patients with diabetes and recent worsening heart
failure. N Engl J Med. 2021; 384:117–128. PMID:
33200892.
23. Felker GM. Building the foundation for a new era of quadruple therapy in heart
failure. Circulation. 2020; 141:112–114. PMID:
31736333.
24. Berliner D, Bauersachs J. Current drug therapy in chronic heart failure: the new guidelines of the
European Society of Cardiology (ESC). Korean Circ J. 2017; 47:543–554. PMID:
28955380.
25. Vaduganathan M, Claggett BL, Jhund PS, et al. Estimating lifetime benefits of comprehensive disease-modifying
pharmacological therapies in patients with heart failure with reduced ejection fraction:
a comparative analysis of three randomised controlled trials. Lancet. 2020; 396:121–128. PMID:
32446323.
26. Shim CY. Heart failure with preserved ejection fraction: the major unmet need in
cardiology. Korean Circ J. 2020; 50:1051–1061. PMID:
33150751.
27. Kim MS, Lee JH, Kim EJ, et al. Korean guidelines for diagnosis and management of chronic heart
failure. Korean Circ J. 2017; 47:555–643. PMID:
28955381.
28. Solomon SD, McMurray JJ, Anand IS, et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection
fraction. N Engl J Med. 2019; 381:1609–1620. PMID:
31475794.
29. Solomon SD, Vaduganathan M, L Claggett B, et al. Sacubitril/valsartan across the spectrum of ejection fraction in heart
failure. Circulation. 2020; 141:352–361. PMID:
31736342.
30. Vaduganathan M, Jhund PS, Claggett BL, et al. A putative placebo analysis of the effects of sacubitril/valsartan in heart
failure across the full range of ejection fraction. Eur Heart J. 2020; 41:2356–2362. PMID:
32221596.
31. Zelniker TA, Braunwald E. Clinical benefit of cardiorenal effects of sodium-glucose cotransporter 2
inhibitors: JACC state-of-the-art review. J Am Coll Cardiol. 2020; 75:435–447. PMID:
32000956.
32. Butler J, Packer M, Greene SJ, et al. Heart failure end points in cardiovascular outcome trials of sodium glucose
cotransporter 2 inhibitors in patients with type 2 diabetes mellitus: a critical
evaluation of clinical and regulatory issues. Circulation. 2019; 140:2108–2118. PMID:
31841369.
33. Patorno E, Pawar A, Franklin JM, et al. Empagliflozin and the risk of heart failure hospitalization in routine
clinical care. Circulation. 2019; 139:2822–2830. PMID:
30955357.
34. Scheen AJ. Cardiovascular effects of new oral glucose-lowering agents: DPP-4 and
SGLT-2 inhibitors. Circ Res. 2018; 122:1439–1459. PMID:
29748368.
35. Anker SD, Butler J, Filippatos G, et al. Baseline characteristics of patients with heart failure with preserved
ejection fraction in the EMPEROR-Preserved trial. Eur J Heart Fail. 2020; 22:2383–2392. PMID:
33251670.
36. Packer M, Butler J, Filippatos G, et al. Design of a prospective patient-level pooled analysis of two parallel
trials of empagliflozin in patients with established heart failure. Eur J Heart Fail. 2020; 22:2393–2398. PMID:
33251659.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download