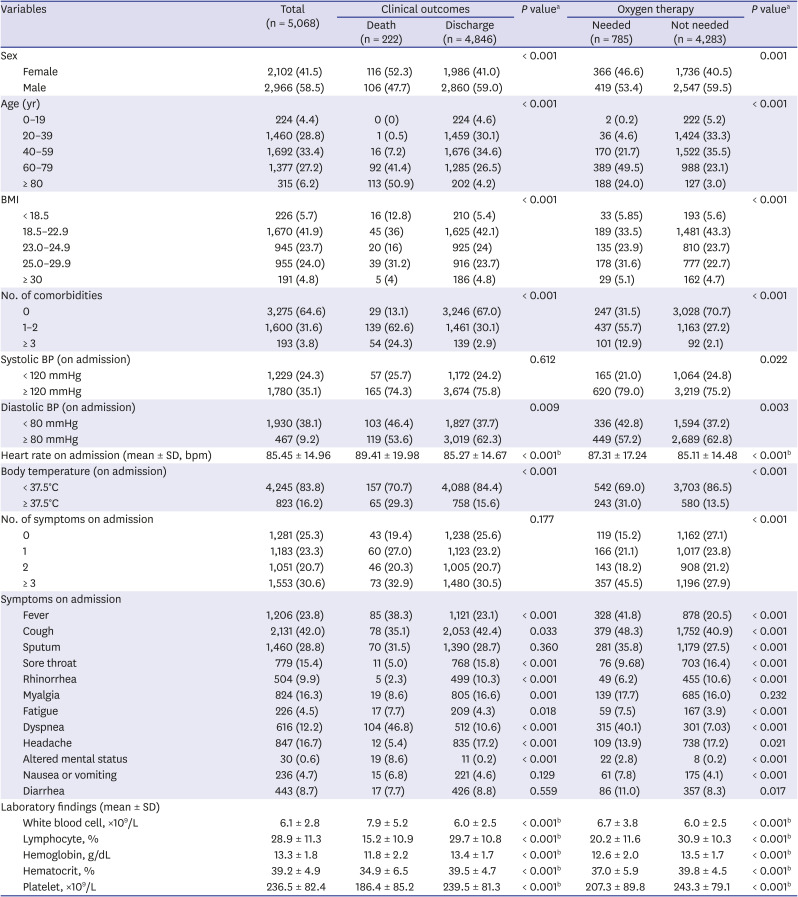
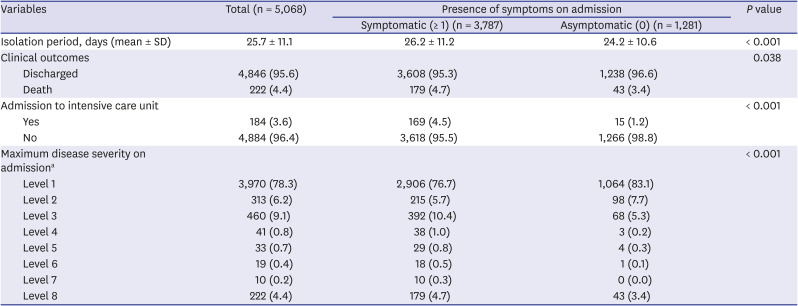
Demographic and clinical characteristics of COVID-19 patients
Table 1 shows the demographic and clinical characteristics of COVID-19 patients included in the study according to their clinical outcomes and the presence of oxygen therapy. Among the 5,068 patients with COVID-19, 555 died and 4,846 were discharged alive. Compared to the proportion of those who survived, a greater proportion of those who died from COVID-19 were female; aged over 80 years; had one or more comorbidities and a higher heart rate; and had a BMI, diastolic blood pressure, and/or body temperature of > 18 kg/m
2, ≤ 80 mmHg, and ≥ 36.5°C, respectively. A higher number of patients had fever, fatigue, dyspnea, or altered mental status at the time of admission among those who died compared to the surviving group. On the other hand, the non-survivor group showed a lower incidence of cough, sore throat, rhinorrhea, myalgia, and headache as early symptoms than those who survived. Laboratory findings showed that white blood cell counts were higher while lymphocyte, hemoglobin, hematocrit, and platelet counts were lower in the non-survivor group than in the survivor group.
Table 1
Demographic and clinical characteristics
|
Variables |
Total (n = 5,068) |
Clinical outcomes |
P valuea
|
Oxygen therapy |
P valuea
|
|
Death (n = 222) |
Discharge (n = 4,846) |
Needed (n = 785) |
Not needed (n = 4,283) |
|
Sex |
|
|
|
< 0.001 |
|
|
0.001 |
|
Female |
2,102 (41.5) |
116 (52.3) |
1,986 (41.0) |
366 (46.6) |
1,736 (40.5) |
|
Male |
2,966 (58.5) |
106 (47.7) |
2,860 (59.0) |
419 (53.4) |
2,547 (59.5) |
|
Age (yr) |
|
|
|
< 0.001 |
|
|
< 0.001 |
|
0–19 |
224 (4.4) |
0 (0) |
224 (4.6) |
2 (0.2) |
222 (5.2) |
|
20–39 |
1,460 (28.8) |
1 (0.5) |
1,459 (30.1) |
36 (4.6) |
1,424 (33.3) |
|
40–59 |
1,692 (33.4) |
16 (7.2) |
1,676 (34.6) |
170 (21.7) |
1,522 (35.5) |
|
60–79 |
1,377 (27.2) |
92 (41.4) |
1,285 (26.5) |
389 (49.5) |
988 (23.1) |
|
≥ 80 |
315 (6.2) |
113 (50.9) |
202 (4.2) |
188 (24.0) |
127 (3.0) |
|
BMI |
|
|
|
< 0.001 |
|
|
< 0.001 |
|
< 18.5 |
226 (5.7) |
16 (12.8) |
210 (5.4) |
33 (5.85) |
193 (5.6) |
|
18.5–22.9 |
1,670 (41.9) |
45 (36) |
1,625 (42.1) |
189 (33.5) |
1,481 (43.3) |
|
23.0–24.9 |
945 (23.7) |
20 (16) |
925 (24) |
135 (23.9) |
810 (23.7) |
|
25.0–29.9 |
955 (24.0) |
39 (31.2) |
916 (23.7) |
178 (31.6) |
777 (22.7) |
|
≥ 30 |
191 (4.8) |
5 (4) |
186 (4.8) |
29 (5.1) |
162 (4.7) |
|
No. of comorbidities |
|
|
|
< 0.001 |
|
|
< 0.001 |
|
0 |
3,275 (64.6) |
29 (13.1) |
3,246 (67.0) |
247 (31.5) |
3,028 (70.7) |
|
1–2 |
1,600 (31.6) |
139 (62.6) |
1,461 (30.1) |
437 (55.7) |
1,163 (27.2) |
|
≥ 3 |
193 (3.8) |
54 (24.3) |
139 (2.9) |
101 (12.9) |
92 (2.1) |
|
Systolic BP (on admission) |
|
|
|
0.612 |
|
|
0.022 |
|
< 120 mmHg |
1,229 (24.3) |
57 (25.7) |
1,172 (24.2) |
165 (21.0) |
1,064 (24.8) |
|
≥ 120 mmHg |
1,780 (35.1) |
165 (74.3) |
3,674 (75.8) |
620 (79.0) |
3,219 (75.2) |
|
Diastolic BP (on admission) |
|
|
|
0.009 |
|
|
0.003 |
|
< 80 mmHg |
1,930 (38.1) |
103 (46.4) |
1,827 (37.7) |
336 (42.8) |
1,594 (37.2) |
|
≥ 80 mmHg |
467 (9.2) |
119 (53.6) |
3,019 (62.3) |
449 (57.2) |
2,689 (62.8) |
|
Heart rate on admission (mean ± SD, bpm) |
85.45 ± 14.96 |
89.41 ± 19.98 |
85.27 ± 14.67 |
< 0.001b
|
87.31 ± 17.24 |
85.11 ± 14.48 |
< 0.001b
|
|
Body temperature (on admission) |
|
|
|
< 0.001 |
|
|
< 0.001 |
|
< 37.5°C |
4,245 (83.8) |
157 (70.7) |
4,088 (84.4) |
542 (69.0) |
3,703 (86.5) |
|
≥ 37.5°C |
823 (16.2) |
65 (29.3) |
758 (15.6) |
243 (31.0) |
580 (13.5) |
|
No. of symptoms on admission |
|
|
|
0.177 |
|
|
< 0.001 |
|
0 |
1,281 (25.3) |
43 (19.4) |
1,238 (25.6) |
119 (15.2) |
1,162 (27.1) |
|
1 |
1,183 (23.3) |
60 (27.0) |
1,123 (23.2) |
166 (21.1) |
1,017 (23.8) |
|
2 |
1,051 (20.7) |
46 (20.3) |
1,005 (20.7) |
143 (18.2) |
908 (21.2) |
|
≥ 3 |
1,553 (30.6) |
73 (32.9) |
1,480 (30.5) |
357 (45.5) |
1,196 (27.9) |
|
Symptoms on admission |
|
|
|
|
|
|
|
|
Fever |
1,206 (23.8) |
85 (38.3) |
1,121 (23.1) |
< 0.001 |
328 (41.8) |
878 (20.5) |
< 0.001 |
|
Cough |
2,131 (42.0) |
78 (35.1) |
2,053 (42.4) |
0.033 |
379 (48.3) |
1,752 (40.9) |
< 0.001 |
|
Sputum |
1,460 (28.8) |
70 (31.5) |
1,390 (28.7) |
0.360 |
281 (35.8) |
1,179 (27.5) |
< 0.001 |
|
Sore throat |
779 (15.4) |
11 (5.0) |
768 (15.8) |
< 0.001 |
76 (9.68) |
703 (16.4) |
< 0.001 |
|
Rhinorrhea |
504 (9.9) |
5 (2.3) |
499 (10.3) |
< 0.001 |
49 (6.2) |
455 (10.6) |
< 0.001 |
|
Myalgia |
824 (16.3) |
19 (8.6) |
805 (16.6) |
0.001 |
139 (17.7) |
685 (16.0) |
0.232 |
|
Fatigue |
226 (4.5) |
17 (7.7) |
209 (4.3) |
0.018 |
59 (7.5) |
167 (3.9) |
< 0.001 |
|
Dyspnea |
616 (12.2) |
104 (46.8) |
512 (10.6) |
< 0.001 |
315 (40.1) |
301 (7.03) |
< 0.001 |
|
Headache |
847 (16.7) |
12 (5.4) |
835 (17.2) |
< 0.001 |
109 (13.9) |
738 (17.2) |
0.021 |
|
Altered mental status |
30 (0.6) |
19 (8.6) |
11 (0.2) |
< 0.001 |
22 (2.8) |
8 (0.2) |
< 0.001 |
|
Nausea or vomiting |
236 (4.7) |
15 (6.8) |
221 (4.6) |
0.129 |
61 (7.8) |
175 (4.1) |
< 0.001 |
|
Diarrhea |
443 (8.7) |
17 (7.7) |
426 (8.8) |
0.559 |
86 (11.0) |
357 (8.3) |
0.017 |
|
Laboratory findings (mean ± SD) |
|
|
|
|
|
|
|
|
White blood cell, ×109/L |
6.1 ± 2.8 |
7.9 ± 5.2 |
6.0 ± 2.5 |
< 0.001b
|
6.7 ± 3.8 |
6.0 ± 2.5 |
< 0.001b
|
|
Lymphocyte, % |
28.9 ± 11.3 |
15.2 ± 10.9 |
29.7 ± 10.8 |
< 0.001b
|
20.2 ± 11.6 |
30.9 ± 10.3 |
< 0.001b
|
|
Hemoglobin, g/dL |
13.3 ± 1.8 |
11.8 ± 2.2 |
13.4 ± 1.7 |
< 0.001b
|
12.6 ± 2.0 |
13.5 ± 1.7 |
< 0.001b
|
|
Hematocrit, % |
39.2 ± 4.9 |
34.9 ± 6.5 |
39.5 ± 4.7 |
< 0.001b
|
37.0 ± 5.9 |
39.8 ± 4.5 |
< 0.001b
|
|
Platelet, ×109/L |
236.5 ± 82.4 |
186.4 ± 85.2 |
239.5 ± 81.3 |
< 0.001b
|
207.3 ± 89.8 |
243.3 ± 79.1 |
< 0.001b
|
Of the 5,068 patients included in the study, 785 required supplemental oxygen therapy and 4,283 did not. Compared to those who did not require oxygen support, a greater proportion of individuals who required oxygen support were predominantly female; aged 60–79 years; had one or more comorbidities and higher heart rates; and a systolic blood pressure, diastolic blood pressure, and/or body temperature of ≥ 120 mmHg, ≤ 80 mmHg, and ≥ 36.5°C, respectively. Among the symptoms on admission, fever, cough, sputum, fatigue, dyspnea, altered mental status, nausea or vomiting, and diarrhea were more prevalent in the group requiring supplemental oxygen than in the group not requiring oxygen support. Conversely, fewer individuals with oxygen therapy had sore throat and rhinorrhea at the time of admission compared to those without the need for oxygen therapy. Laboratory findings showed that more patients needing oxygen support had higher white blood cell, lymphocyte, hemoglobin, hematocrit, and platelet counts than those without oxygen support.
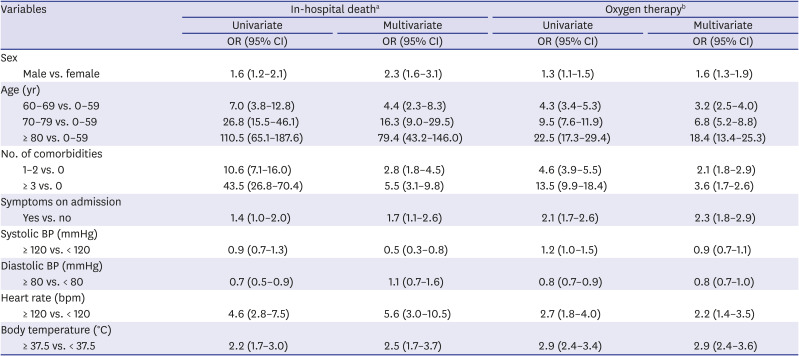
Influencing factors of in-hospital death and supplemental oxygen therapy
Table 3 shows risk factors associated with in-hospital death and severe cases that required supplemental oxygen therapy within COVID-19 patients. According to the results from univariate analyses, male sex, older age, presence of comorbidities, presence of symptoms on admission, lower diastolic blood pressure, higher heart rate, and higher body temperature were associated with higher crude odds of death and a severer state needing oxygen therapy. In addition, those who had systolic blood pressure of 120 mmHg or higher had a greater likelihood of requiring supplemental oxygen. In multivariate analyses, male sex, older age, higher number of comorbidities, presence of symptoms on admission, heart rate 120 bpm or higher, and body temperature 37.5°C or higher resulted in greater odds of in-hospital death and oxygen therapy.
Table 3
Risk factors associated with in-hospital death and severe cases requiring oxygen therapy
|
Variables |
In-hospital deatha
|
Oxygen therapyb
|
|
Univariate |
Multivariate |
Univariate |
Multivariate |
|
OR (95% CI) |
OR (95% CI) |
OR (95% CI) |
OR (95% CI) |
|
Sex |
|
|
|
|
|
Male vs. female |
1.6 (1.2–2.1) |
2.3 (1.6–3.1) |
1.3 (1.1–1.5) |
1.6 (1.3–1.9) |
|
Age (yr) |
|
|
|
|
|
60–69 vs. 0–59 |
7.0 (3.8–12.8) |
4.4 (2.3–8.3) |
4.3 (3.4–5.3) |
3.2 (2.5–4.0) |
|
70–79 vs. 0–59 |
26.8 (15.5–46.1) |
16.3 (9.0–29.5) |
9.5 (7.6–11.9) |
6.8 (5.2–8.8) |
|
≥ 80 vs. 0–59 |
110.5 (65.1–187.6) |
79.4 (43.2–146.0) |
22.5 (17.3–29.4) |
18.4 (13.4–25.3) |
|
No. of comorbidities |
|
|
|
|
|
1–2 vs. 0 |
10.6 (7.1–16.0) |
2.8 (1.8–4.5) |
4.6 (3.9–5.5) |
2.1 (1.8–2.9) |
|
≥ 3 vs. 0 |
43.5 (26.8–70.4) |
5.5 (3.1–9.8) |
13.5 (9.9–18.4) |
3.6 (1.7–2.6) |
|
Symptoms on admission |
|
|
|
|
|
Yes vs. no |
1.4 (1.0–2.0) |
1.7 (1.1–2.6) |
2.1 (1.7–2.6) |
2.3 (1.8–2.9) |
|
Systolic BP (mmHg) |
|
|
|
|
|
≥ 120 vs. < 120 |
0.9 (0.7–1.3) |
0.5 (0.3–0.8) |
1.2 (1.0–1.5) |
0.9 (0.7–1.1) |
|
Diastolic BP (mmHg) |
|
|
|
|
|
≥ 80 vs. < 80 |
0.7 (0.5–0.9) |
1.1 (0.7–1.6) |
0.8 (0.7–0.9) |
0.8 (0.7–1.0) |
|
Heart rate (bpm) |
|
|
|
|
|
≥ 120 vs. < 120 |
4.6 (2.8–7.5) |
5.6 (3.0–10.5) |
2.7 (1.8–4.0) |
2.2 (1.4–3.5) |
|
Body temperature (°C) |
|
|
|
|
|
≥ 37.5 vs. < 37.5 |
2.2 (1.7–3.0) |
2.5 (1.7–3.7) |
2.9 (2.4–3.4) |
2.9 (2.4–3.6) |
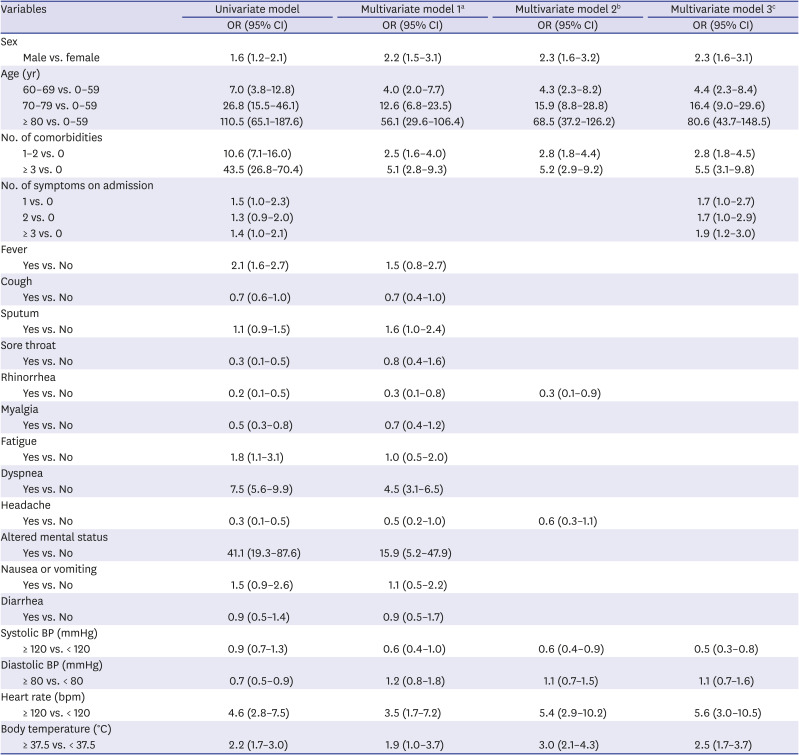
Table 4 displays the results of the logistic regression analyses on the association between the presence of early symptoms and in-hospital death in COVID-19 patients. In the univariate analysis, male sex, age 60 years or older, prevalence of one or more comorbidities, and having a single symptom on admission were related to higher odds of death during hospitalization compared to female sex, age below 60 years, no comorbidity, and asymptomatic status. In addition, those with fever, fatigue, dyspnea, altered mental status, heart rate 120 bpm or higher, and body temperature 37.5°C or higher resulted in greater crude odds of in-hospital death, while cough, sore throat, rhinorrhea, myalgia, headache, and diastolic blood pressure 80 mmHg or higher resulted in lower crude odds of death in hospital.
Table 4
Early symptoms associated with in-hospital death
|
Variables |
Univariate model |
Multivariate model 1a
|
Multivariate model 2b
|
Multivariate model 3c
|
|
OR (95% CI) |
OR (95% CI) |
OR (95% CI) |
OR (95% CI) |
|
Sex |
|
|
|
|
|
Male vs. female |
1.6 (1.2–2.1) |
2.2 (1.5–3.1) |
2.3 (1.6–3.2) |
2.3 (1.6–3.1) |
|
Age (yr) |
|
|
|
|
|
60–69 vs. 0–59 |
7.0 (3.8–12.8) |
4.0 (2.0–7.7) |
4.3 (2.3–8.2) |
4.4 (2.3–8.4) |
|
70–79 vs. 0–59 |
26.8 (15.5–46.1) |
12.6 (6.8–23.5) |
15.9 (8.8–28.8) |
16.4 (9.0–29.6) |
|
≥ 80 vs. 0–59 |
110.5 (65.1–187.6) |
56.1 (29.6–106.4) |
68.5 (37.2–126.2) |
80.6 (43.7–148.5) |
|
No. of comorbidities |
|
|
|
|
|
1–2 vs. 0 |
10.6 (7.1–16.0) |
2.5 (1.6–4.0) |
2.8 (1.8–4.4) |
2.8 (1.8–4.5) |
|
≥ 3 vs. 0 |
43.5 (26.8–70.4) |
5.1 (2.8–9.3) |
5.2 (2.9–9.2) |
5.5 (3.1–9.8) |
|
No. of symptoms on admission |
|
|
|
|
|
1 vs. 0 |
1.5 (1.0–2.3) |
|
|
1.7 (1.0–2.7) |
|
2 vs. 0 |
1.3 (0.9–2.0) |
|
|
1.7 (1.0–2.9) |
|
≥ 3 vs. 0 |
1.4 (1.0–2.1) |
|
|
1.9 (1.2–3.0) |
|
Fever |
|
|
|
|
|
Yes vs. No |
2.1 (1.6–2.7) |
1.5 (0.8–2.7) |
|
|
|
Cough |
|
|
|
|
|
Yes vs. No |
0.7 (0.6–1.0) |
0.7 (0.4–1.0) |
|
|
|
Sputum |
|
|
|
|
|
Yes vs. No |
1.1 (0.9–1.5) |
1.6 (1.0–2.4) |
|
|
|
Sore throat |
|
|
|
|
|
Yes vs. No |
0.3 (0.1–0.5) |
0.8 (0.4–1.6) |
|
|
|
Rhinorrhea |
|
|
|
|
|
Yes vs. No |
0.2 (0.1–0.5) |
0.3 (0.1–0.8) |
0.3 (0.1–0.9) |
|
|
Myalgia |
|
|
|
|
|
Yes vs. No |
0.5 (0.3–0.8) |
0.7 (0.4–1.2) |
|
|
|
Fatigue |
|
|
|
|
|
Yes vs. No |
1.8 (1.1–3.1) |
1.0 (0.5–2.0) |
|
|
|
Dyspnea |
|
|
|
|
|
Yes vs. No |
7.5 (5.6–9.9) |
4.5 (3.1–6.5) |
|
|
|
Headache |
|
|
|
|
|
Yes vs. No |
0.3 (0.1–0.5) |
0.5 (0.2–1.0) |
0.6 (0.3–1.1) |
|
|
Altered mental status |
|
|
|
|
|
Yes vs. No |
41.1 (19.3–87.6) |
15.9 (5.2–47.9) |
|
|
|
Nausea or vomiting |
|
|
|
|
|
Yes vs. No |
1.5 (0.9–2.6) |
1.1 (0.5–2.2) |
|
|
|
Diarrhea |
|
|
|
|
|
Yes vs. No |
0.9 (0.5–1.4) |
0.9 (0.5–1.7) |
|
|
|
Systolic BP (mmHg) |
|
|
|
|
|
≥ 120 vs. < 120 |
0.9 (0.7–1.3) |
0.6 (0.4–1.0) |
0.6 (0.4–0.9) |
0.5 (0.3–0.8) |
|
Diastolic BP (mmHg) |
|
|
|
|
|
≥ 80 vs. < 80 |
0.7 (0.5–0.9) |
1.2 (0.8–1.8) |
1.1 (0.7–1.5) |
1.1 (0.7–1.6) |
|
Heart rate (bpm) |
|
|
|
|
|
≥ 120 vs. < 120 |
4.6 (2.8–7.5) |
3.5 (1.7–7.2) |
5.4 (2.9–10.2) |
5.6 (3.0–10.5) |
|
Body temperature (°C) |
|
|
|
|
|
≥ 37.5 vs. < 37.5 |
2.2 (1.7–3.0) |
1.9 (1.0–3.7) |
3.0 (2.1–4.3) |
2.5 (1.7–3.7) |
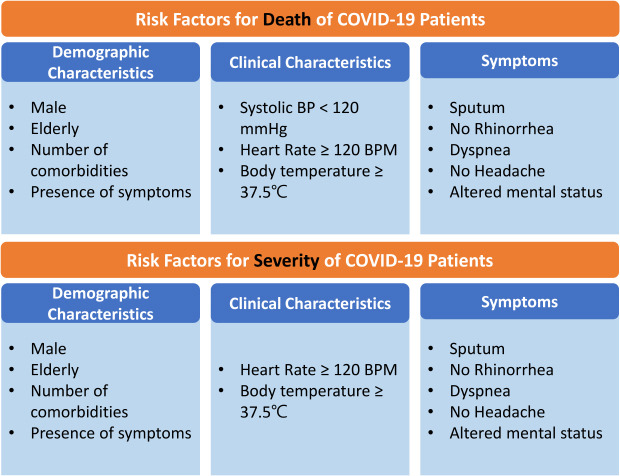
In the multivariate analysis, after adjusting for sex, age, number of comorbidities, and vital signs on admission, patients with sputum (odds ratio [OR], 1.6; 95% confidence interval [CI], 1.0–2.4), dyspnea (OR, 4.5; 95% CI, 3.1–6.5), and altered mental status (OR, 15.9; 95% CI, 5.2–47.9) had higher odds of death, while patients with rhinorrhea (OR, 0.3; 95% CI, 0.1–0.8) and headache (OR, 0.5; 95% CI, 0.2–1.0) had lower odds of death. When rhinorrhea and headache were separately analyzed as independent variables and adjusted for sex, age, number of comorbidities, vital signs on admission, odds of death were lower in patients with rhinorrhea (OR, 0.3; 95% CI, 0.1–0.9) while insignificantly associated with headache at the point of admission. In the analysis with the number of symptoms on admission as the independent variable, a higher number of symptoms on admission resulted in higher odds of death, with the presence of one symptom resulted in 1.7-fold (95% CI, 1.0–2.7), two symptoms in 1.7-fold (95% CI, 1.0–2.9), and three or more symptoms 1.9-fold (95% CI, 1.2–3.0) higher mortality in patients. In all three multivariate models, patients had higher odds of death if they were male, aged 60 years or older, had one or more comorbidities, had systolic blood pressure 120 mmHg or lower, and heart rate 120 bpm or higher.
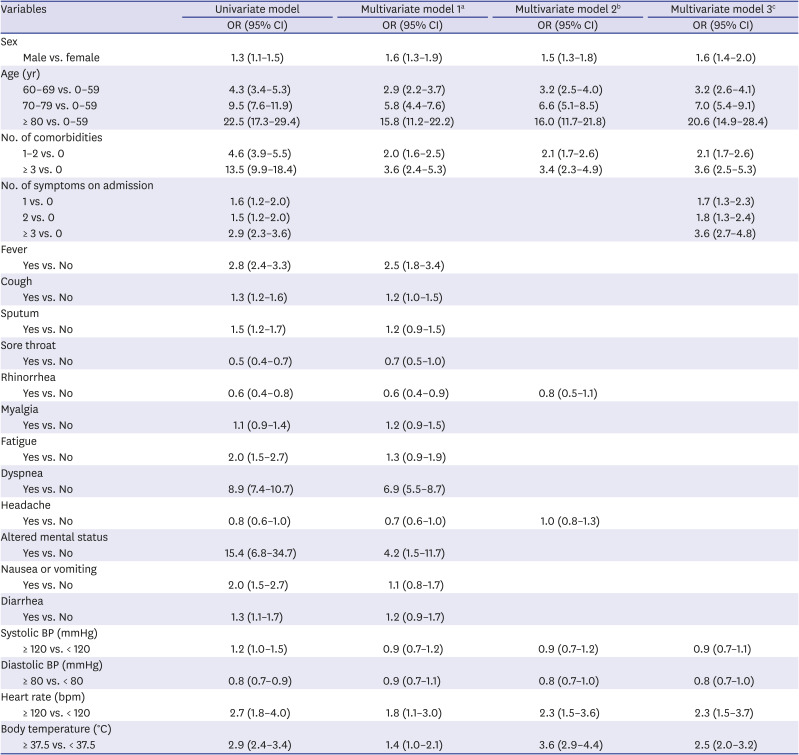
Table 5 shows the results of the logistic regression analyses on the association between the presence of early symptoms and severe illness requiring supplemental oxygen therapy. Results from the univariate analysis showed that male sex, age 60 years or older, prevalence of one or more comorbidities, and having one or more symptoms on admission were related to higher odds in more severe states requiring supplemental oxygen during hospitalization, compared to female sex, age below 60 years, no comorbidity, and asymptomatic status. In addition, those with fever, cough, sputum, fatigue, dyspnea, altered mental status, nausea or vomiting, systolic blood pressure 120 mmHg or higher, heart rate 120 bpm or higher, and body temperature 37.5°C or higher resulted in higher crude odds of disease severity level 3 or higher (i.e., in need of oxygen therapy) during hospitalization. In contrast, sore throat, rhinorrhea, headache, and diastolic blood pressure 80 mmHg or higher resulted in the opposite of lower crude odds to be critical patient in need of ventilation.
Table 5
Early symptoms associated with severe cases requiring oxygen therapy
|
Variables |
Univariate model |
Multivariate model 1a
|
Multivariate model 2b
|
Multivariate model 3c
|
|
OR (95% CI) |
OR (95% CI) |
OR (95% CI) |
OR (95% CI) |
|
Sex |
|
|
|
|
|
Male vs. female |
1.3 (1.1–1.5) |
1.6 (1.3–1.9) |
1.5 (1.3–1.8) |
1.6 (1.4–2.0) |
|
Age (yr) |
|
|
|
|
|
60–69 vs. 0–59 |
4.3 (3.4–5.3) |
2.9 (2.2–3.7) |
3.2 (2.5–4.0) |
3.2 (2.6–4.1) |
|
70–79 vs. 0–59 |
9.5 (7.6–11.9) |
5.8 (4.4–7.6) |
6.6 (5.1–8.5) |
7.0 (5.4–9.1) |
|
≥ 80 vs. 0–59 |
22.5 (17.3–29.4) |
15.8 (11.2–22.2) |
16.0 (11.7–21.8) |
20.6 (14.9–28.4) |
|
No. of comorbidities |
|
|
|
|
|
1–2 vs. 0 |
4.6 (3.9–5.5) |
2.0 (1.6–2.5) |
2.1 (1.7–2.6) |
2.1 (1.7–2.6) |
|
≥ 3 vs. 0 |
13.5 (9.9–18.4) |
3.6 (2.4–5.3) |
3.4 (2.3–4.9) |
3.6 (2.5–5.3) |
|
No. of symptoms on admission |
|
|
|
|
|
1 vs. 0 |
1.6 (1.2–2.0) |
|
|
1.7 (1.3–2.3) |
|
2 vs. 0 |
1.5 (1.2–2.0) |
|
|
1.8 (1.3–2.4) |
|
≥ 3 vs. 0 |
2.9 (2.3–3.6) |
|
|
3.6 (2.7–4.8) |
|
Fever |
|
|
|
|
|
Yes vs. No |
2.8 (2.4–3.3) |
2.5 (1.8–3.4) |
|
|
|
Cough |
|
|
|
|
|
Yes vs. No |
1.3 (1.2–1.6) |
1.2 (1.0–1.5) |
|
|
|
Sputum |
|
|
|
|
|
Yes vs. No |
1.5 (1.2–1.7) |
1.2 (0.9–1.5) |
|
|
|
Sore throat |
|
|
|
|
|
Yes vs. No |
0.5 (0.4–0.7) |
0.7 (0.5–1.0) |
|
|
|
Rhinorrhea |
|
|
|
|
|
Yes vs. No |
0.6 (0.4–0.8) |
0.6 (0.4–0.9) |
0.8 (0.5–1.1) |
|
|
Myalgia |
|
|
|
|
|
Yes vs. No |
1.1 (0.9–1.4) |
1.2 (0.9–1.5) |
|
|
|
Fatigue |
|
|
|
|
|
Yes vs. No |
2.0 (1.5–2.7) |
1.3 (0.9–1.9) |
|
|
|
Dyspnea |
|
|
|
|
|
Yes vs. No |
8.9 (7.4–10.7) |
6.9 (5.5–8.7) |
|
|
|
Headache |
|
|
|
|
|
Yes vs. No |
0.8 (0.6–1.0) |
0.7 (0.6–1.0) |
1.0 (0.8–1.3) |
|
|
Altered mental status |
|
|
|
|
|
Yes vs. No |
15.4 (6.8–34.7) |
4.2 (1.5–11.7) |
|
|
|
Nausea or vomiting |
|
|
|
|
|
Yes vs. No |
2.0 (1.5–2.7) |
1.1 (0.8–1.7) |
|
|
|
Diarrhea |
|
|
|
|
|
Yes vs. No |
1.3 (1.1–1.7) |
1.2 (0.9–1.7) |
|
|
|
Systolic BP (mmHg) |
|
|
|
|
|
≥ 120 vs. < 120 |
1.2 (1.0–1.5) |
0.9 (0.7–1.2) |
0.9 (0.7–1.2) |
0.9 (0.7–1.1) |
|
Diastolic BP (mmHg) |
|
|
|
|
|
≥ 80 vs. < 80 |
0.8 (0.7–0.9) |
0.9 (0.7–1.1) |
0.8 (0.7–1.0) |
0.8 (0.7–1.0) |
|
Heart rate (bpm) |
|
|
|
|
|
≥ 120 vs. < 120 |
2.7 (1.8–4.0) |
1.8 (1.1–3.0) |
2.3 (1.5–3.6) |
2.3 (1.5–3.7) |
|
Body temperature (°C) |
|
|
|
|
|
≥ 37.5 vs. < 37.5 |
2.9 (2.4–3.4) |
1.4 (1.0–2.1) |
3.6 (2.9–4.4) |
2.5 (2.0–3.2) |
In the multivariate analysis, after adjusting for sex, age, number of comorbidities, and vital signs on admission, patients with fever (OR, 2.5; 95% CI, 1.8–3.4), dyspnea (OR, 6.9, 95% CI, 5.5–8.7), and altered mental status (OR, 4.2; 95% CI, 1.5–11.7) had higher odds of needing supplemental oxygen, while patients with rhinorrhea (OR, 0.6; 95% CI, 0.4–0.9), and headache (OR, 0.7; 95% CI, 0.6–1.0) had lower odds of being in such a state. When rhinorrhea and headache were separately analyzed as independent variables and adjusted for sex, age, number of comorbidities, vital signs on admission and odds of severe state in need of oxygen therapy were insignificantly associated with both rhinorrhea and headache at the point of admission. In the analysis with the number of symptoms on admission as the independent variable, a higher number of symptoms on admission resulted in higher odds of a severe state requiring oxygen therapy, with the presence of one symptom resulting in 1.7-fold (95% CI, 1.3–2.3), two symptoms in 1.8-fold (95% CI, 1.3–2.4), and three or more symptoms in 3.6-fold (95% CI, 2.7–4.8) higher mortality in patients. In all three multivariate models, patients who were male, aged 60 or older, had one or more comorbidities, had a heart rate of 120 bpm or higher, and whose body temperature was 37.5°C or higher had greater odds of severe illness with a need for supplemental oxygen therapy.
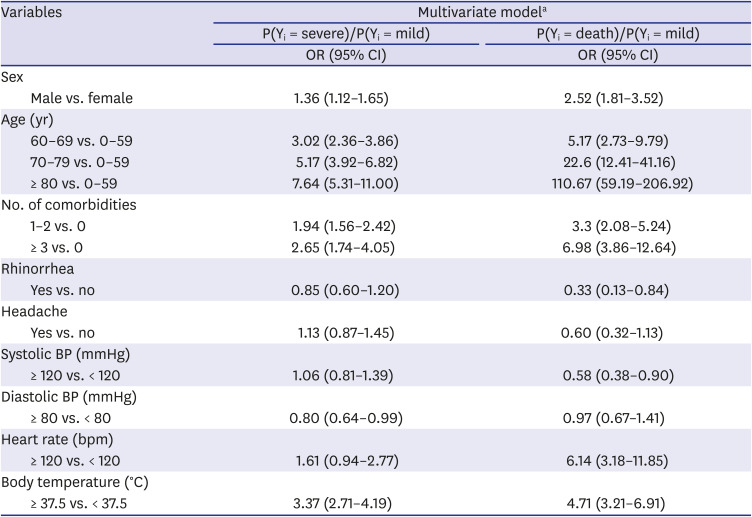
Table 6 presents the results of the polychotomous logistic analysis. The maximum severity on admission was considered as the outcome variable, and was used to categorize mildly affected, severely affected, and death patients. Mildly affected patients were defined as those who did not require oxygen therapy (levels 1–2), while severely affected patients were defined as those who required oxygen therapy (levels 3–7). The results obtained were similar to those obtained during dichotomous logistic analyses. Male sex, age ≥ 60 years, prevalence of one or more comorbidities, diastolic blood pressure ≤ 80 mmHg, and body temperature ≥ 36.5°C were related to a higher severity of the disease. Furthermore, the male sex, age ≥ 60 years, prevalence of one or more comorbidities, absence of rhinorrhea, systolic blood pressure ≤ 120 mmHg, heart rate ≥ 120 bpm, and body temperature ≥ 36.5°C were associated with a higher risk of death.
Table 6
Polychotomous logistic analysis for early symptoms associated with coronavirus disease 2019 severity
|
Variables |
Multivariate modela
|
|
P(Yi = severe)/P(Yi = mild) |
P(Yi = death)/P(Yi = mild) |
|
OR (95% CI) |
OR (95% CI) |
|
Sex |
|
|
|
Male vs. female |
1.36 (1.12–1.65) |
2.52 (1.81–3.52) |
|
Age (yr) |
|
|
|
60–69 vs. 0–59 |
3.02 (2.36–3.86) |
5.17 (2.73–9.79) |
|
70–79 vs. 0–59 |
5.17 (3.92–6.82) |
22.6 (12.41–41.16) |
|
≥ 80 vs. 0–59 |
7.64 (5.31–11.00) |
110.67 (59.19–206.92) |
|
No. of comorbidities |
|
|
|
1–2 vs. 0 |
1.94 (1.56–2.42) |
3.3 (2.08–5.24) |
|
≥ 3 vs. 0 |
2.65 (1.74–4.05) |
6.98 (3.86–12.64) |
|
Rhinorrhea |
|
|
|
Yes vs. no |
0.85 (0.60–1.20) |
0.33 (0.13–0.84) |
|
Headache |
|
|
|
Yes vs. no |
1.13 (0.87–1.45) |
0.60 (0.32–1.13) |
|
Systolic BP (mmHg) |
|
|
|
≥ 120 vs. < 120 |
1.06 (0.81–1.39) |
0.58 (0.38–0.90) |
|
Diastolic BP (mmHg) |
|
|
|
≥ 80 vs. < 80 |
0.80 (0.64–0.99) |
0.97 (0.67–1.41) |
|
Heart rate (bpm) |
|
|
|
≥ 120 vs. < 120 |
1.61 (0.94–2.77) |
6.14 (3.18–11.85) |
|
Body temperature (°C) |
|
|
|
≥ 37.5 vs. < 37.5 |
3.37 (2.71–4.19) |
4.71 (3.21–6.91) |










 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download