INTRODUCTION
Asthma is not only a common chronic disease but also one of the major causes of hospitalization in children. Asthma should be managed appropriately especially in children for preventing progression to airway remodeling and impaired lung function. Early accurate diagnosis and appropriate treatment enables children to enjoy a high quality of life and leads to much better disease control and outcomes.
Asthma may differ in prevalence and severity by race, region, and country. Thus, physicians should have appropriate guidance upon data with the prevalence of accurate asthma in the general population of children.
In most previous epidemiological studies, questionnaires based on subjective symptoms and past medical history of asthma have been used for estimating the prevalence of asthma.
The International Study of Asthma and Allergies of Childhood (ISAAC) questionnaire is a widely accepted standardized tool for evaluating asthma prevalence.
1 However, the questionnaire alone may overestimate the true prevalence of asthma, since asthma-mimicking symptoms can manifest in various other diseases. Therefore, it is important to estimate accurate asthma prevalence based on an objective method such as the bronchial provocation test in a general population of children.
Bronchial hyperresponsiveness (BHR) is one of the key features of asthma and is demonstrated in almost all subjects with current symptomatic asthma.
2 Defining current asthma with BHR and recent wheezing in the past 12 months have been considered useful in evaluating asthma prevalence in the general population.
3
In pediatric populations, the prevalence of BHR with “Current asthma symptoms” varies among studies. The prevalence of BHR to methacholine (provocative concentration of methacholine causing a 20% reduction in forced expiratory volume in one second [FEV1]; PC20 ≤ 16 mg/mL) in children who had wheezing in the past 12 months has been reported to range from 25% in the United States
4 to 60% in Turkey
5 which is a large difference. In addition, the frequency of BHR in current wheezing within 12 months showed considerable inconsistency in Korean pediatric population studies: 6%
6 vs. 56%.
7 As above, BHR differs from country to country, and even in the same country, it may vary depending on the region and age.
In Korean studies, epidemiological data on the nationwide prevalence of all ages in elementary school students were insufficient. Some large-scaled studies of asthma prevalence among Korean children were mainly based on questionnaires. There have been a few studies on BHR in children.
678 Subjects in those studies did not include all ages of elementary students. A study that conducted a methacholine challenge test (MCT) on subjects aged 7–19 years with asthma symptoms reported 4.6% prevalence of asthma in 1997.
7 As healthcare environments, including advances in therapeutic agents, early diagnosis, as well as early and proper treatment, have changed from the mid-1990s when previous studies were conducted, it is necessary to update asthma prevalence in Korean children. Therefore, this is a meaningful study to understand the trend in prevalence of asthma over time.
The aim of this study was to assess the prevalence of current asthma and BHR using both the ISAAC questionnaire and MCT in Korean elementary school students in the metropolitan cities of Incheon, Gwangju (southern inland), and Gyeonggi.
DISCUSSION
The purpose of this study was to investigate the prevalence of “Current asthma” with the ISAAC questionnaire and MCT in Korean elementary school students.
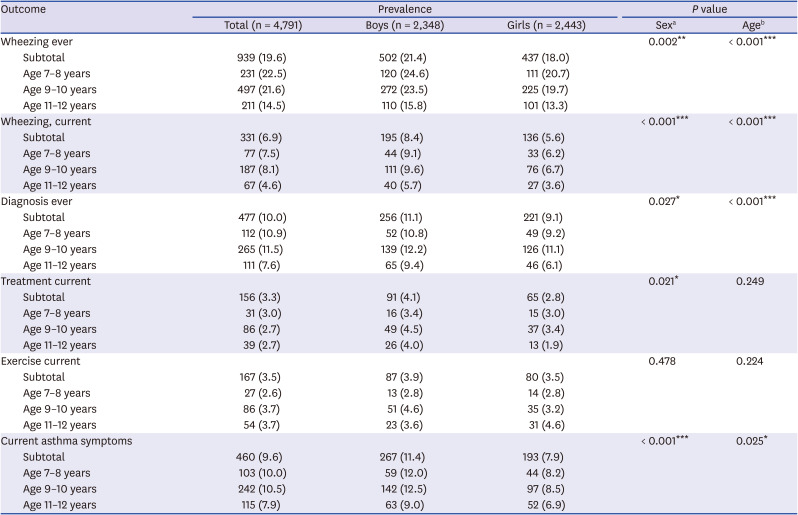
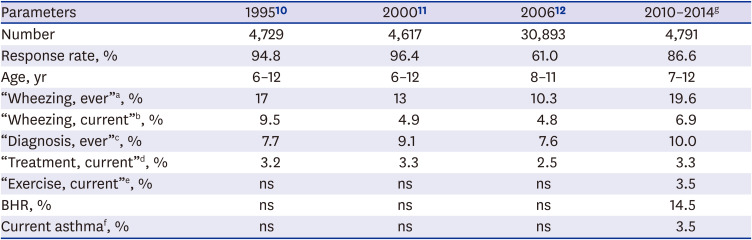
The study results showed that the prevalence of “Wheezing, ever,” “Wheezing, current,” “Diagnosis, ever,” “Treatment, current,” and "Exercise, current” was 19.6%, 6.9%, 10.0%, 3.3% and 3.5%, respectively in Korean students (age 7–12 years). “Current asthma symptoms” defined as positive response to “Wheezing, current,” “Treatment, current,” or “Exercise, current” was 9.6%. However, among them, 26% of the children had BHR in this cross-sectional study. The reason why students with BHR were low in subjects with current asthma symptoms may be because their symptoms were mild, have no recent symptoms in the previous week, or have been misdiagnosed. In addition, the Korean version of ISAAC questionnaire might have low sensitivity to diagnose asthma. In a study that conducted the asthma ISAAC questionnaire and hypertonic saline bronchial challenge test for 13–14-year-old Korean children, the sensitivity of BHR to “asthma ever” was the highest at 0.61, wheezing after exercise 0.35, nocturnal wheezing 0.24.
13 Overall, the sensitivity of the ISAAC questionnaire to BHR was low. These results are consistent with our findings, suggesting that the ISSAC survey questions are not sufficient to predict BHR. Therefore, these results imply that the usefulness of the Korean ISAAC questionnaire for diagnosing current asthma in Korean children should be reevaluated.
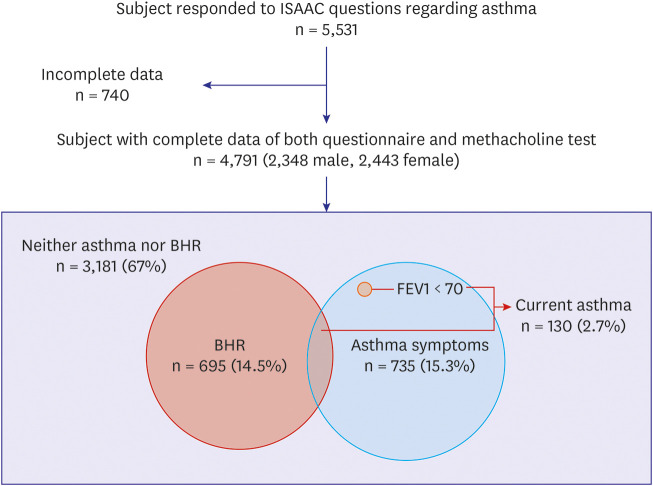
We defined “Current asthma” if the subjects with “Current asthma symptoms” showed BHR on the MCT or had less than 70% of predicted FEV1 value. The prevalence of “Current asthma” in the current study was 2.7%, consistent with 2.5% of the Seoul study (current asthma, lifetime diagnosis of asthma and experience of wheezing within 12 months),
14 but 4.6% in a survey conducted about 20 years ago,
7 suggesting decrement of true current asthma.
The time trends in asthma symptom prevalence have shown geographic/national differences. The prevalence of “Current asthma symptoms” in children have reduced in Western countries and Oceania, where the prevalence was previously high. On the other hand, the prevalence of “Wheezing, current” increased in parts of Africa, Latin America, and Asia, where the prevalence was previously low. Consequently, international differences in asthma symptom prevalence have reduced.
15
In Korean children, the prevalence of asthma symptoms based on questionnaire showed a decreasing trend from 1995 to 2006, however, the study results increased compared to previous 2006 data (
Table 6). The previous studies were based on questionnaire survey only, and the 2006 study had a low response rate compared to the current study. Therefore, previous studies have limitations in predicting the true prevalence of asthma.
Our study approached the prevalence of “Current asthma” more accurately using both the ISAAC questionnaire and MCT. The current study also included a relatively large number of children. Therefore, the prevalence of asthma can be estimated more accurately than those in previous studies.
The prevalence of BHR can vary in studies due to the different study designs including different study subjects, bronchial provocation stimuli or their cutoff value.
816171819
The MCT is highly sensitive and widely used to detect and quantify BHR.
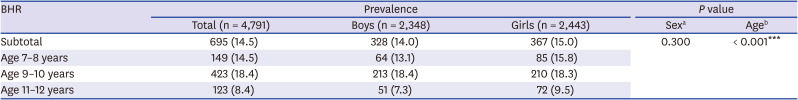
20 The prevalence of BHR defined as methacholine PC20 ≤ 16 mg/mL was 14.5% in children aged 7–12 years (mean age, 9.6 years) in our study, which was lower than those in previous studies of Korean children with similar study periods; 19.4% (mean age, 9.2 years) in 2012
8 and 17.5% (age 6–15 years) from 2009 to 2012.
21 However, the study was conducted only at 1 school, which is located in Seoul.
8 The other study subjects were also in Seoul.
21 However, the present study populations were in Incheon, Gwangju (southern inland), and Gyeonggi, which were different from previous studies.
A German study showed that the prevalence of BHR increased from 6.4% in 1992–1993 to 11.6% in 1995–1996 in East German children.
22 It is suggested that changes in the environment, such as Western lifestyle, attending daycare center, exposure to new allergens and/or air pollutants contributed to the increase in BHR of children for a short period of time after reunification of East Germany.
2324
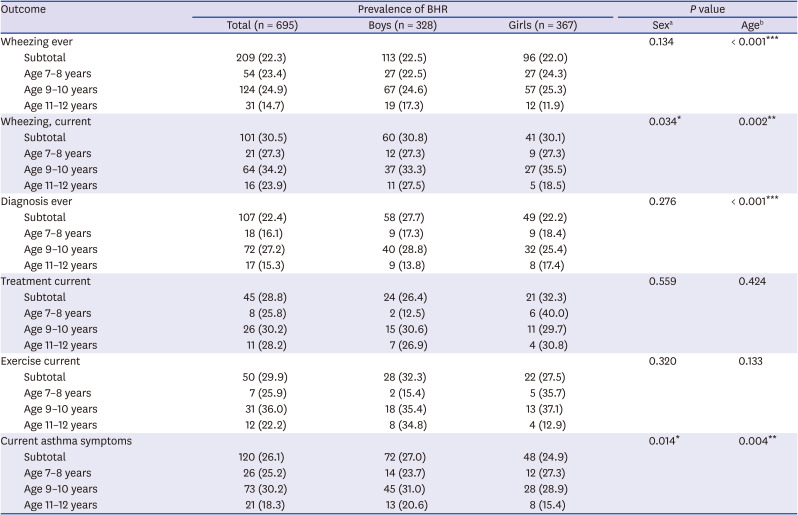
There have been few epidemiological studies of BHR in Korean children with asthma symptoms. The present study found that the prevalence of BHR was 30.5%, 28.8%, and 29.9% in children with “Wheezing, current,” “Treatment, current,” and “Exercise, current,” respectively.
The present study result of 26% BHR in children with “Current asthma symptoms” was consistent with 20.5% (age 7–19 years)
7 and 20.1% (age 10–12 years)
6 in subjects with asthma symptoms in Korean epidemiological studies, which were low, altogether.
In contrast to epidemiological studies, clinical studies reported BHR prevalence of 84.0% in Korean children (mean age, 8.7 years) who had asthma symptoms or were diagnosed with asthma
21 and 70.7% in Northern Taiwan children (mean age, 7.5 years) who had symptoms within the 12 months,
25 which showed that there was a strong correlation between asthma symptoms and BHR.
In our study, the prevalence of BHR and asthma symptoms in younger age groups (age 7–8 years and 9–10 years) was higher than those in the 11–12-year age groups, showing significant correlation with age (
Table 2). Many studies have suggested that younger children are more sensitive to bronchoprovocation tests.
826 One of the reasons is that the airway structure, impedance, and contractile force of the bronchial smooth muscle change with age, reducing airway hypersensitivity as the airways grows in size. Lung function in children continues to increase until adolescence.
27
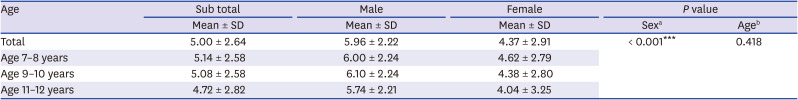
The prevalence of BHR was also correlated with sex and was more prevalent in boys before puberty onset in this study. Physiological function or maturation of the lungs during infancy differs by sex, which extends to pre-puberty, adolescence and adulthood. The prevalence of BHR by sex varies with age. Since the growth spurt begins earlier in girls than in boys, BHR drops earlier in girls compared to boys.
28 The current study found that the prevalence of BHR decreased sharply in boys from 11 years of age and from 9 years of age in girls, consistent with findings that prevalence of BHR and asthma in boys decreases with age after puberty and that of asthma in girls increases from puberty.
29 As females have increased asthma prevalence starting around puberty, the male sex predominance of asthma and BHR is reversed at puberty onset.
30 The mechanisms underlying the sex difference in the prevalence of asthma and BHR are still unknown. Anatomical and hormonal factors are thought to be differently involved in the development of airways between males and females from birth to puberty.
313233 Our study included pre-pubertal children, so males showed a higher prevalence of BHR.
This study has several limitations. First, we defined BHR as methacholine PC20 ≤ 16 mg/mL without stratifying age groups. Since BHR could be influenced by age, the cutoff values of methacholine PC20 to define BHR may need to be adjusted by age.
21 However, the BHR positive criteria was proposed as PC20 ≤ 16 mg/mL
9 and considering the age range from 7 to 12 years of this study, the criterion of BHR can be accepted. Second, the study subjects were not national but limited in Incheon, Gwangju, and Gyeonggi areas, and the subject groups were not evenly distributed, especially, age 9–10 years was greater in Incheon, which had higher prevalence of asthma symptoms and BHR. However, the 2006 nationwide data showed that the prevalence of asthma (“Wheezing, current”) was almost similar in Incheon, Gyeonggi and Gwangju, which were slightly more than that in Seoul.
34 Third, the MCT may not be appropriate for diagnosing exercise-induced bronchoconstriction (EIB) compare to field exercise challenge test.
3135 However, there was less chance of underdiagnosis of current asthma, because the prevalence of EIB from this study was only 3.5%, and about one third of EIB showed BHR. Fourth, although there might have been some patients who had negative BHR in response to asthma medication within 12 months, the number of subjects who had been treated for asthma within the last 12 months was very small, 156 out of total 4,791 (3.3%). In addition, several studies have consistently raised the issue of low adherence to asthma medications.
363738 According to a recent Korean study, adherence to asthma medication was low (38% of inhalant users, 50% of oral users and 67% of transdermal patch).
39 Therefore, given that the treatment group was very small and the adherence to asthma medications was low, the effect of treatment on the asthma prevalence would be insignificant. Finally, the prevalence of asthma symptoms was highest in the 9–10 year-olds in the current study, but it was expected to be highest in the 7–8-year-olds, making it difficult to identify trend with age. In this study, the number of subjects in the 9–10-year-old group was remarkably large (n = 2,304), and that in the 7–8-year-old group (n = 1,027) was the least. In addition, there was a difference in number among regions. The number of subjects in Incheon, Gwangju, and Gyeonggi was 2,421, 1,029, and 1,341, respectively. Therefore, the reason for the high prevalence of asthma symptoms and BHR in age 9–10 years is thought to be that more children were included in the region (Incheon) with a relatively high prevalence.
BHR is an important feature that reflects airway hyperresponsiveness in asthma. Transient or persistent BHR can also occur in exposure to environmental pollutants and irritants, viral respiratory infections, chronic infections, rhinitis, sarcoidosis, mitral valve stenosis, and tracheal dysplasia.
40 The positive rate of BHR (22.3%) in children with “Diagnosis, ever” was similar in those with “Wheezing, ever” (22.4%). There may be some reasons why the proportion of BHR was low in children with “Diagnosis ever.” First, cases of early transient wheezer could be included. Second, healthcare environment in Korea including Health Insurance Review and Assessment Service requires asthma diagnosis when doctors prescribe asthma-related medications, such as inhaled corticosteroids, leukotriene receptor antagonist. Third, early and appropriate treatment in early childhood asthma may have contributed to resolution of BHR. Finally, there might be a possibility of overdiagnosis since the understanding of wheezing in the asthma-related questionnaire may have been poorly understood depending on the individuals.
In conclusion, these study results showed that about one third of children with asthma symptoms had BHR, and overall prevalence of current asthma was 2.7%. Most nationwide epidemiological studies of Korean children are based on questionnaires only, and studies conducted with both questionnaires and bronchial provocation test have limitations of small number of population and/or local regions. The current large-scaled study with both questionnaires and MCT provides accurate current asthma prevalence in Korean elementary school children. This would help physicians diagnose asthma appropriately. Considering that performing MCT is time and energy consuming and not easy, especially for children, we suggest measuring BHR selectively in children with asthma symptoms. Since, about one third of children with asthma symptoms based on ISAAC questionnaire are likely to be true asthmatics, studies are necessary to develop modified questionnaire which have high diagnostic accuracy of asthma.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download