CASE DESCRIPTION
A previously healthy 55-year-old Korean female was diagnosed with COVID-19 in Mexico. She complained of fever and cough for 2 days, and the diagnosis was confirmed on the day of hospital admission (day 1) by real-time polymerase chain reaction (RT-PCR) test (
Fig. 1). Despite medical treatments including antibiotics, dexamethasone, and oxygen supplement, COVID-19-associated ARDS developed and mechanical ventilation with prone positioning was applied on day 4. (When the patient developed COVID-19, there were no recommendations for Remdesivir, Tocilizumab, and Baricitinib and these medications were not administered.) However, the patient's condition continued to decline, and venovenous extracorporeal membrane oxygenation (ECMO) was initiated on day 39. She had been closely observed for the possible recovery of ARDS, but there were no signs of clinical improvement for more than 7 weeks. She was therefore recommended for lung transplant and was transferred to our institution in Korea by air ambulance on day 55.
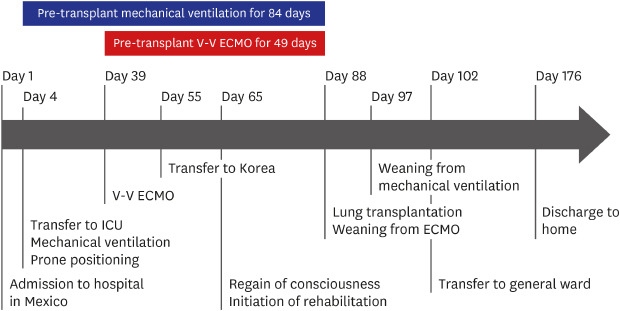
Fig. 1
Timeline of the patient's clinical course.
Note that prolonged pre-transplant mechanical ventilation for 84 days and V-V ECMO for 49 days.
ICU = intensive care unit, V-V ECMO = venovenous extracorporeal membrane oxygenation.
During the air transportation, the patient was supported with venovenous ECMO with femoro-jugular configuration using the ultracompact Cardiohelp® system (Maquet Cardiopulmonary AG, Hirrlingen, Germany). An intensivist, a critical care nurse, and a perfusionist from the sending hospital, who have sufficient experience in managing critically ill patients, participated in the transportation, which took more than 24 hours from Mexico to Korea. The air ambulance was equipped with the devices needed to manage the patient safely and adequately, including mechanical ventilation and ECMO. Since 2 months had passed after the initial diagnosis of COVID-19 and the RT-PCR tests had repeatedly revealed negative results, the isolation and precaution procedure during transportation was categorized as a “suspected case,” rather than a “confirmed case.”
At the time of admission to our intensive care unit (ICU), she was dependent on both mechanical ventilation (mode, pressure control ventilation; fraction of inspired oxygen, 1.0; positive end-expiratory pressure, 10 cmH2O; inspiratory pressure, 20 cmH2O; respiratory rate, 16/min; inspiration time, 1.07 sec; and tidal volume 116 mL) and venovenous ECMO with femoro-jugular configuration (blood flow 4.0 L/min and sweep gas flow 5 L/min). The transthoracic echocardiography revealed a D-shaped left ventricle with decreased right ventricular contractility causing hemodynamic instability. She initially required moderate doses of vasopressors (norepinephrine, 0.3 mcg/kg/min; vasopressin, 0.02 unit/min; and epinephrine, 0.04 mcg/kg/min), but they were tapered gradually and were stopped 3 days later with meticulous titration for mechanical ventilation and volume status. As the hemodynamic status stabilized, the level of sedation was reduced, and she regained consciousness on day 65. The patient was then rehabilitated according to the institution's protocol. Although she had been sedated and bed-ridden for more than 2 months, she could perform “sit to stand up training” at bedside after 3 weeks of rehabilitation (day 86).
The initial RT-PCR test in our institution revealed a positive result, but the cycle threshold (Ct) value was as high as 35. Tests were then performed twice a week according to the institution's protocol; it turned negative 6 days later (day 61) and had been remained in that state thereafter. Despite the initial positive result at our institution, the isolation and precaution policy was kept as a “suspected case” because the prolonged time from symptom onset and the high Ct value suggested a high probability of no actual infectivity. Isolation was lifted 10 days later when the RT-PCR tests revealed 2 consecutive negative results.
Meanwhile, she was assessed for lung transplant by an institutional multidisciplinary lung transplant committee, including cardiothoracic surgeons, pulmonologists, intensivists, infectious disease specialists, anesthesiologists, and radiologists. After a thorough review of medical records, the committee judged that the patient was unlikely to recover and decided to list her for lung transplant based on the following considerations: 1) despite the best treatment for ARDS, there had been no clinical improvement for more than 2 months; 2) the lung compliance was severely reduced for several weeks, suggesting end-stage fibrosis; 3) the radiologic images persistently showed parenchymal infiltrations and fibrotic changes throughout the whole lung (
Fig. 2); 4) the patient was young and there was no other organ failure; 5) the patient was awakened and is physically capable of performing rehabilitation; 6) there was no available treatment option other than lung transplant.
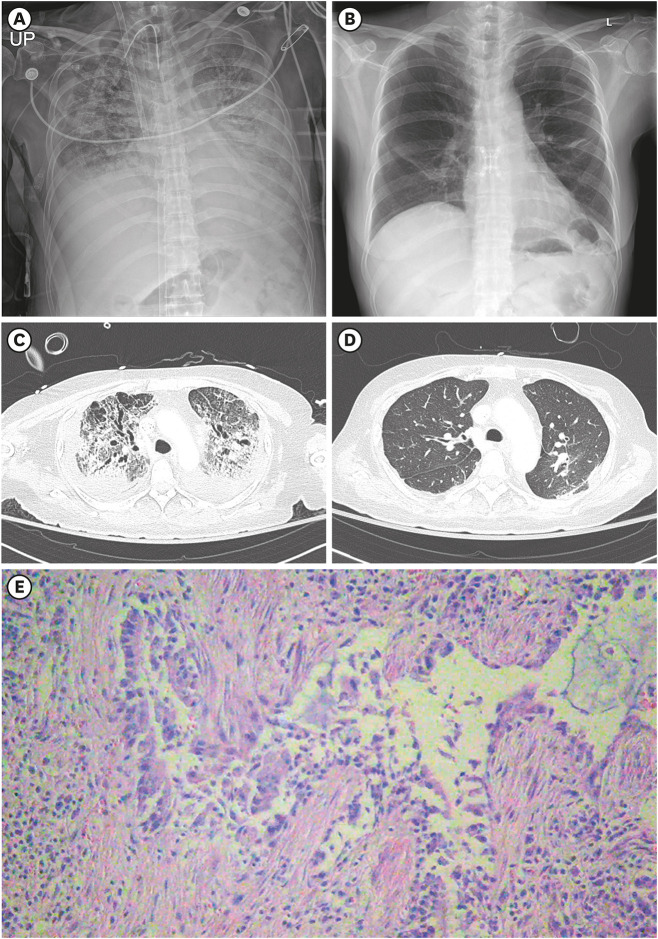
Fig. 2
Radiological images and pathological finding of the lungs.
(A) Chest X-rays of the patient on day 87, a day before lung transplantation and (B) on day 175 (postoperative day 87), a day before hospital discharge. (C) Chest computed tomography scan on day 70 and (D) on day 113 (postoperative day 25). (E) Microscopic image of hematoxylin and eosin staining of explanted lung.
On day 88, 49 days after the initiation of ECMO support, donor lungs became available. Given the negative RT-PCR tests for > 2 weeks, transplant was prepared according to the same profile as patients without COVID-19. Sequential double-lung transplant was performed with trans-sternal lateral anterior thoracotomy. There was a focal adhesion requiring adhesiolysis on the left lung, but the pneumonectomy was successfully performed without complication. The right lung was first anastomosed in order of bronchus, pulmonary vein, and pulmonary artery. The left lung was then transplanted sequentially. The pre-transplant venovenous ECMO was switched to central ECMO for hemodynamic support during transplant and was successfully weaned at the end of operation. The total operation time was 491 minutes, including 238 minutes of pump time. The cold and warm ischemic times for the right lung were 136 and 63 minutes, respectively, and 245 and 74 minutes, respectively, for the left lung.
The explanted lungs showed diffuse fibrotic and hemorrhagic changes with patchy areas of gray-white consolidation. Under microscopic examination, they revealed marked infiltration of inflammatory cells with bronchial epithelial damage and subepithelial fibroblastic proliferation, indicating diffuse alveolar damage with end-stage fibrosis (
Fig. 2).
Postoperative management was conducted at the medical ICU in line with the institution’s protocol, which has been described in detail elsewhere.
3 There were no bleeding complications or primary graft dysfunction within 72 hours of transplant. RT-PCR tests were regularly performed post-transplant to confirm the absence of viral reactivation that can potentially arise from high doses of immunosuppressants, and they revealed negative results. Rehabilitation resumed, and the patient was able to stand up with assistance on postoperative day 4. She was liberated from mechanical ventilation on the 9th postoperative day and was transferred to the general ward 5 days later. At 3 months post-transplantation, she was discharged to home without complication (
Fig. 2).
DISCUSSION
This case report details a rare strategy for lung transplant in a patient with treatment-refractory COVID-19-associated ARDS. Although lung transplant has been suggested as a salvage therapy for carefully selected patients with ARDS,
2 there is limited experience on this potentially life-saving procedure for COVID-19-associated ARDS. Our experience provides a valuable confirmation that lung transplant is feasible and may be considered salvage therapy in patients with treatment-refractory COVID-19-associated ARDS.
We faced several challenges when preparing this patient for lung transplant. Firstly, the possibility of transporting a critically ill patient over such a long distance was uncertain. Several studies had shown the feasibility of international air transportation with mechanical circulatory support
4; however, we could not find any reports on transportation with ECMO across continents. To the best of our knowledge, this is the first report to demonstrate the feasibility of inter-continent air transportation of a critically ill patient requiring ECMO support.
In addition, the composition of medical staffs and the devices used in this case may be of reference in the future studies. In the Extracorporeal Life Support Organization (ELSO) guidelines, the recommended transport team consists of a cannulating physician, a surgical assistant, an ECMO physician, an ECMO specialist, and a transport nurse or respiratory therapist. In addition, extra staffs may be required in the long-haul intercontinental transport. However, it is also necessary to consider that there is not enough space within the aircraft used for the transport. Therefore, the ECMO team, particularly participated in the inter-continent air transport, should be formed with highly skilled professions to ensure patient safety with minimal manpower. Furthermore, it cannot be emphasized enough that it is important to assure that enough supplies, medications, oxygen, etc., are available for the whole transport which may take more than 24 hours. The amount of oxygen brought should cover for a significant time delay and increase in patient oxygen demand.
An additional challenge that we had faced in considering whether to perform lung transplant for ARDS was assessing the potential for regeneration of the damaged lungs.
2 Given the potential for recovery of ARDS even after prolonged treatment,
5 the candidate for lung transplant should be carefully reviewed by the multidisciplinary committee.
6 In addition, a sufficient period of observation is required before the decision for lung transplant can be made.
2 In the present case, we judged that the patient was unlikely to recover because there had been no clinical improvement for > 2 months. However, it is widely uncertain how long the patient should be observed before lung transplant for COVID-19-associated ARDS. There are only 2 published reports regarding lung transplant for the disease.
78 In a case reported by Lang from Austria, the patient was observed for 52 days before being listed for lung transplant.
7 This is in line with the present case, in which the patient was observed for > 2 months for the potential recovery of ARDS. However, in another case series, including 2 patients from China, they were listed for lung transplant earlier, on day 27 and 33, respectively.
8 Although it is difficult to define the optimal timing for lung transplant from these limited number of reports, it is noteworthy that the candidates were observed for a minimum of 1 or 2 months before lung transplant for COVID-19-associated ARDS in all cases.
On the other hand, clinicians should be aware that the trade-off of prolonged pre-transplant waiting may worsen post-transplant outcomes.
9 Since most candidates for lung transplant are dependent on either mechanical ventilation, ECMO, or both, the prolonged pre-transplant mechanical support may increase the risk of neuromuscular deconditioning, which subsequently contributes to unfavorable clinical outcomes.
10 For example, in a report by Lang and colleagues, the patient was supported with pre-transplant mechanical ventilation and venovenous ECMO for 51 and 45 days, respectively, and because of the severe neuromuscular deconditioning, it took more than 2 months to transfer the patient to a non-ICU setting after transplant.
7 On the contrary, in the present case, despite the longer duration of pre-transplant support with mechanical ventilation for 84 days and venovenous ECMO for 49 days, the patient was vigorously rehabilitated before and after transplant, could be liberated from mechanical ventilation on postoperative day 9, and transferred to the general ward on day 14. We believe that our strategy, which focuses on awakening and rehabilitation during the perioperative period, provides valuable inspiration for the prevention of neuromuscular deconditioning and the subsequent improvement of post-transplant outcomes in patients with COVID-19-associated-ARDS.
Length of detection of COVID-19 and interpretation of the results was another important consideration in patients with ARDS. Since corticosteroids are frequently administrated for COVID-19-associated ARDS,
11 there is a concern that their administration may extend the duration of viral detection.
12 In the present case, the viral nucleic acid was detected until 58 days after the initial diagnosis, comparable with the report by Lang that showed detection up to 68 days.
7 However, in both reports, the Ct values in the late period were very high, suggesting they might have been derived from residual nucleic acid segments, which do not have actual infectivity.
13 Moreover, Lang et al.
7 had confirmed that, despite the positive RT-PCR test, there was no viable virus in Vero cell culture in the late period. Therefore, we agree with the opinion that the patients with COVID-19-associated ARDS should not be excluded from the lung transplant because of the positive RT-PCR test alone, particularly when the Ct value is high and the time elapsed from the symptom onset is more than 1 month.
The final consideration was the possibility of viral reactivation after transplant, contributed by the high doses of immunosuppressants. However, the RT-PCR tests were negative throughout the postoperative period, suggesting once the virus is eradicated, it does not seem to be reactivated even with high doses of immunosuppressants. However, this needs to be confirmed in future studies.
In conclusion, our experience suggests that lung transplant is feasible in a patient with COVID-19-associated ARDS and may be considered a salvage therapy when conventional treatments are refractory. However, several concerns need to be addressed, and subsequent studies are required.