1. Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2016; 68(10):1082–1115. PMID:
27036918.
2. Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the task force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018; 39(3):213–260. PMID:
28886622.
3. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020; 41(3):407–477. PMID:
31504439.
4. Mauri L, Kereiakes DJ, Yeh RW, Driscoll-Shempp P, Cutlip DE, Steg PG, et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med. 2014; 371(23):2155–2166. PMID:
25399658.

5. Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015; 372(19):1791–1800. PMID:
25773268.

6. Hahn JY, Song YB, Oh JH, Chun WJ, Park YH, Jang WJ, et al. Effect of P2Y12 inhibitor monotherapy vs dual antiplatelet therapy on cardiovascular events in patients undergoing percutaneous coronary intervention: the SMART-CHOICE randomized clinical trial. JAMA. 2019; 321(24):2428–2437. PMID:
31237645.
7. Watanabe H, Domei T, Morimoto T, Natsuaki M, Shiomi H, Toyota T, et al. Effect of 1-month dual antiplatelet therapy followed by clopidogrel vs 12-month dual antiplatelet therapy on cardiovascular and bleeding events in patients receiving PCI: the STOPDAPT-2 randomized clinical trial. JAMA. 2019; 321(24):2414–2427. PMID:
31237644.
8. Giustino G, Chieffo A, Palmerini T, Valgimigli M, Feres F, Abizaid A, et al. Efficacy and safety of dual antiplatelet therapy after complex PCI. J Am Coll Cardiol. 2016; 68(17):1851–1864. PMID:
27595509.

9. Cho SW, Park K, Ahn JH, Park TK, Lee SY, Kim J, et al. Extended clopidogrel therapy beyond 12 months and long-term outcomes in patients with diabetes mellitus receiving coronary arterial second-generation drug-eluting stents. Am J Cardiol. 2018; 122(5):705–711. PMID:
30057226.

10. Hahn JY, Song YB, Oh JH, Cho DK, Lee JB, Doh JH, et al. 6-month versus 12-month or longer dual antiplatelet therapy after percutaneous coronary intervention in patients with acute coronary syndrome (SMART-DATE): a randomised, open-label, non-inferiority trial. Lancet. 2018; 391(10127):1274–1284. PMID:
29544699.

11. Mehran R, Baber U, Sharma SK, Cohen DJ, Angiolillo DJ, Briguori C, et al. Ticagrelor with or without aspirin in high-risk patients after PCI. N Engl J Med. 2019; 381(21):2032–2042. PMID:
31556978.

12. Shin DH, Kang HJ, Jang JS, Moon KW, Song YB, Park DW, et al. The current status of percutaneous coronary intervention in Korea: based on year 2014 & 2016 cohort of Korean percutaneous coronary intervention (K-PCI) registry. Korean Circ J. 2019; 49(12):1136–1151. PMID:
31347316.
13. Yeh RW, Secemsky EA, Kereiakes DJ, Normand SL, Gershlick AH, Cohen DJ, et al. Development and validation of a prediction rule for benefit and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA. 2016; 315(16):1735–1749. PMID:
27022822.

14. Baber U, Mehran R, Giustino G, Cohen DJ, Henry TD, Sartori S, et al. Coronary thrombosis and major bleeding after PCI with drug-eluting stents: risk scores from PARIS. J Am Coll Cardiol. 2016; 67(19):2224–2234. PMID:
27079334.
15. Costa F, Adamo M, Ariotti S, Ferrante G, Navarese EP, Leonardi S, et al. Left main or proximal left anterior descending coronary artery disease location identifies high-risk patients deriving potentially greater benefit from prolonged dual antiplatelet therapy duration. EuroIntervention. 2016; 11(11):e1222–30. PMID:
26342472.

16. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third universal definition of myocardial infarction. Eur Heart J. 2012; 33(20):2551–2567. PMID:
22922414.

17. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007; 115(17):2344–2351. PMID:
17470709.
18. Stolker JM, Kennedy KF, Lindsey JB, Marso SP, Pencina MJ, Cutlip DE, et al. Predicting restenosis of drug-eluting stents placed in real-world clinical practice: derivation and validation of a risk model from the EVENT registry. Circ Cardiovasc Interv. 2010; 3(4):327–334. PMID:
20606136.
19. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011; 123(23):2736–2747. PMID:
21670242.
20. Jang WJ, Ahn SG, Song YB, Choi SH, Chun WJ, Oh JH, et al. Benefit of prolonged dual antiplatelet therapy after implantation of drug-eluting stent for coronary bifurcation lesions: results from the coronary bifurcation stenting registry II. Circ Cardiovasc Interv. 2018; 11(7):e005849. PMID:
30006330.

21. Bhatt DL, Steg PG, Mehta SR, Leiter LA, Simon T, Fox K, et al. Ticagrelor in patients with diabetes and stable coronary artery disease with a history of previous percutaneous coronary intervention (THEMIS-PCI): a phase 3, placebo-controlled, randomised trial. Lancet. 2019; 394(10204):1169–1180. PMID:
31484629.
22. Yoshikawa S, Ashikaga T, Miyazaki T, Kurihara K, Hirao K. Long-term efficacy and safety of everolimus-eluting stent implantation in Japanese patients with acute coronary syndrome: five-year real-world data from the Tokyo-MD PCI study. J Interv Cardiol. 2019; 2019:3146848. PMID:
31777468.

23. Armstrong PC, Leadbeater PD, Chan MV, Kirkby NS, Jakubowski JA, Mitchell JA, et al. In the presence of strong P2Y12 receptor blockade, aspirin provides little additional inhibition of platelet aggregation. J Thromb Haemost. 2011; 9(3):552–561. PMID:
21143373.

24. Park TK, Song YB, Ahn J, Carriere KC, Hahn JY, Yang JH, et al. Clopidogrel versus aspirin as an antiplatelet monotherapy after 12-month dual-antiplatelet therapy in the era of drug-eluting stents. Circ Cardiovasc Interv. 2016; 9(1):e002816. PMID:
26755571.

25. Navarese EP, Andreotti F, Schulze V, Kołodziejczak M, Buffon A, Brouwer M, et al. Optimal duration of dual antiplatelet therapy after percutaneous coronary intervention with drug eluting stents: meta-analysis of randomised controlled trials. BMJ. 2015; 350:h1618. PMID:
25883067.

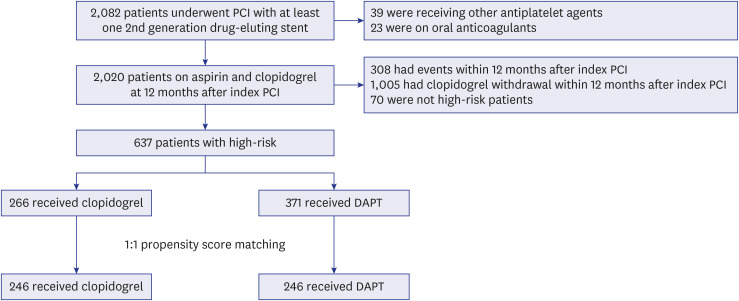
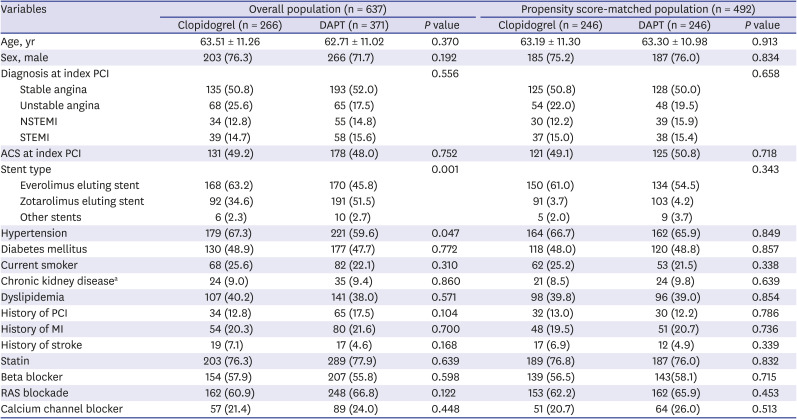
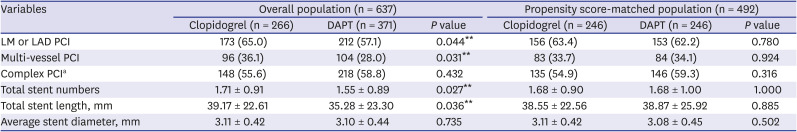

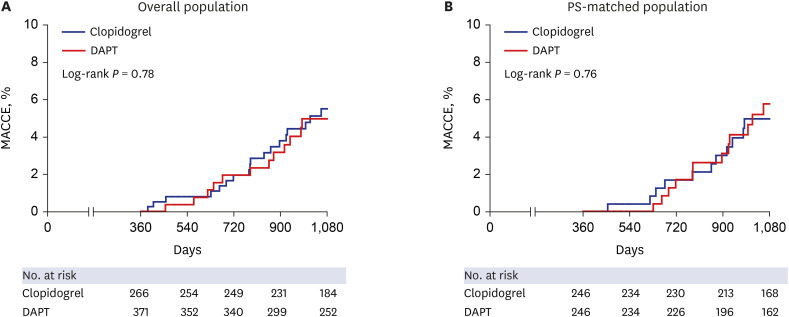





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download