1. Turan S, Turan OM, Miller J, Harman C, Reece EA, Baschat AA. Decreased fetal cardiac performance in the first trimester correlates with hyperglycemia in pregestational maternal diabetes. Ultrasound Obstet Gynecol. 2011; 38:325–31.

2. Passarella G, Trifirò G, Gasparetto M, Moreolo GS, Milanesi O. Disorders in glucidic metabolism and congenital heart diseases: detection and prevention. Pediatr Cardiol. 2013; 34:931–7.

3. Rizzo G, Arduini D, Capponi A, Romanini C. Cardiac and venous blood flow in fetuses of insulin-dependent diabetic mothers: evidence of abnormal hemodynamics in early gestation. Am J Obstet Gynecol. 1995; 173:1775–81.

4. Han SS, Wang G, Jin Y, Ma ZL, Jia WJ, Wu X, et al. Investigating the mechanism of hyperglycemia-induced fetal cardiac hypertrophy. PLoS One. 2015; 10:e0139141.

5. Miranda JO, Cerqueira RJ, Ramalho C, Areias JC, Henriques-Coelho T. Fetal cardiac function in maternal diabetes: a onventional and speckle-tracking echocardiographic study. J Am Soc Echocardiogr. 2018; 31:333–41.
6. Balli S, Pac FA, Ece İ, Oflaz MB, Kibar AE, Kandemir Ö. Assessment of cardiac functions in fetuses of gestational diabetic mothers. Pediatr Cardiol. 2014; 35:30–7.

7. Patey O, Carvalho JS, Thilaganathan B. Perinatal changes in fetal cardiac geometry and function in diabetic pregnancy at term. Ultrasound Obstet Gynecol. 2019; 54:634–42.

8. Mohsin M, Sadqani S, Younus K, Hoodbhoy Z, Ashiqali S, Atiq M. Evaluation of cardiac function in fetuses of mothers with gestational diabetes. Cardiol Young. 2019; 29:1264–7.

9. Rocha LA, Rolo LC, Araujo E Júnior. How to perform a functional assessment of the fetal heart: a pictorial review. Ultrasonography. 2019; 38:365–73.
10. Bravo-Valenzuela NJ, Peixoto AB, Carrilho MC, Siqueira Pontes AL, Chagas CC, Simioni C, et al. Fetal cardiac function by three-dimensional ultrasound using 4D-STIC and VOCAL - an update. J Ultrason. 2019; 19:287–94.

11. Huhta JC. Fetal congestive heart failure. Semin Fetal Neonatal Med. 2005; 10:542–52.

12. Tongsong T, Wanapirak C, Piyamongkol W, Sirichotiyakul S, Tongprasert F, Srisupundit K, et al. Fetal ventricular shortening fraction in hydrops fetalis. Obstet Gynecol. 2011; 117:84–91.

13. Molina FS, Faro C, Sotiriadis A, Dagklis T, Nicolaides KH. Heart stroke volume and cardiac output by four-dimensional ultrasound in normal fetuses. Ultrasound Obstet Gynecol. 2008; 32:181–7.

14. Simioni C, Nardozza LM, Araujo E Júnior, Rolo LC, Terasaka OA, Zamith MM, et al. Fetal cardiac function assessed by spatio-temporal image correlation. Arch Gynecol Obstet. 2011; 284:253–60.

15. Bravo-Valenzuela NJ, Peixoto AB, Nardozza LM, Souza AS, Araujo E Júnior. Applicability and technical aspects of two-dimensional ultrasonography for assessment of fetal heart function. Med Ultrason. 2017; 19:94–101.

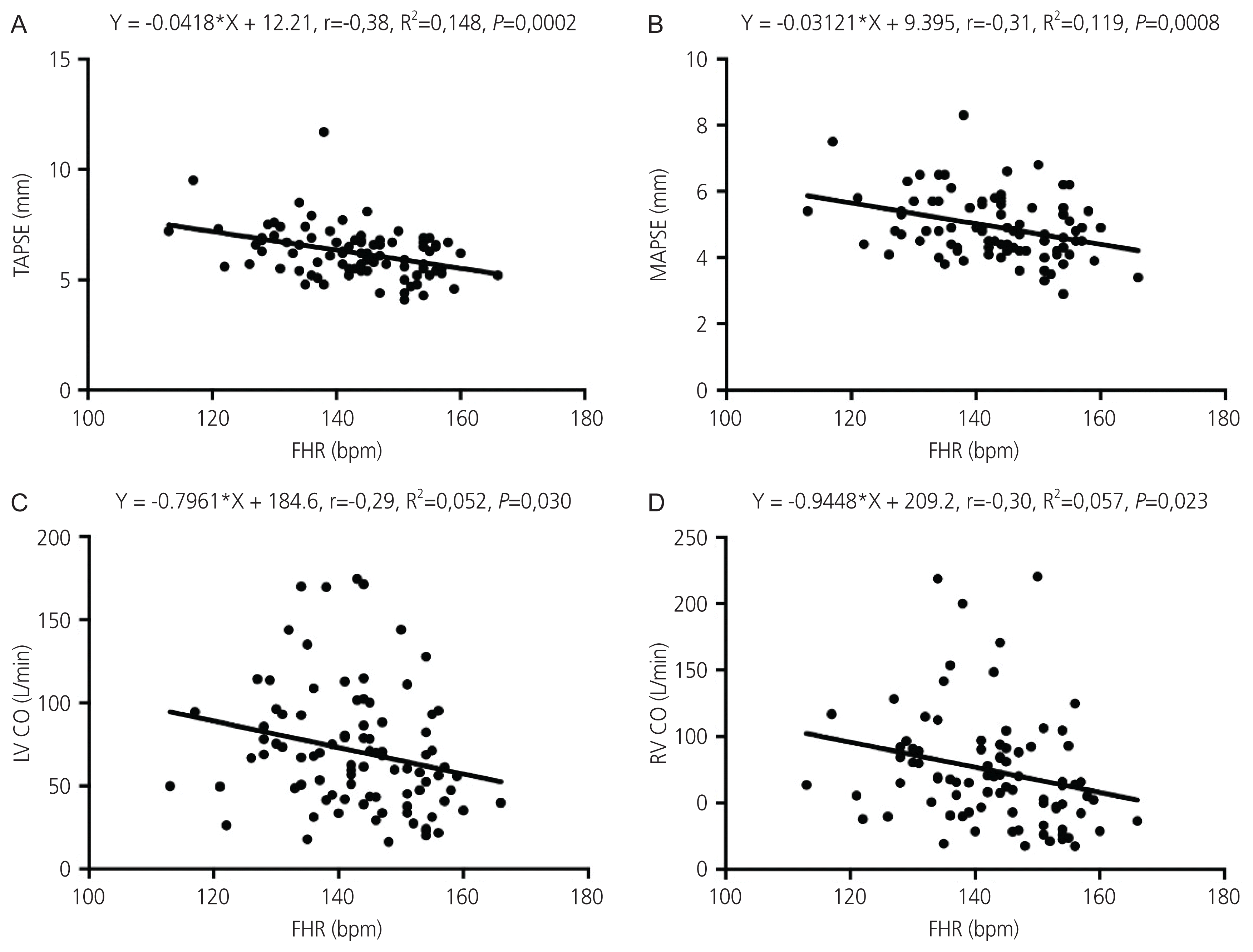
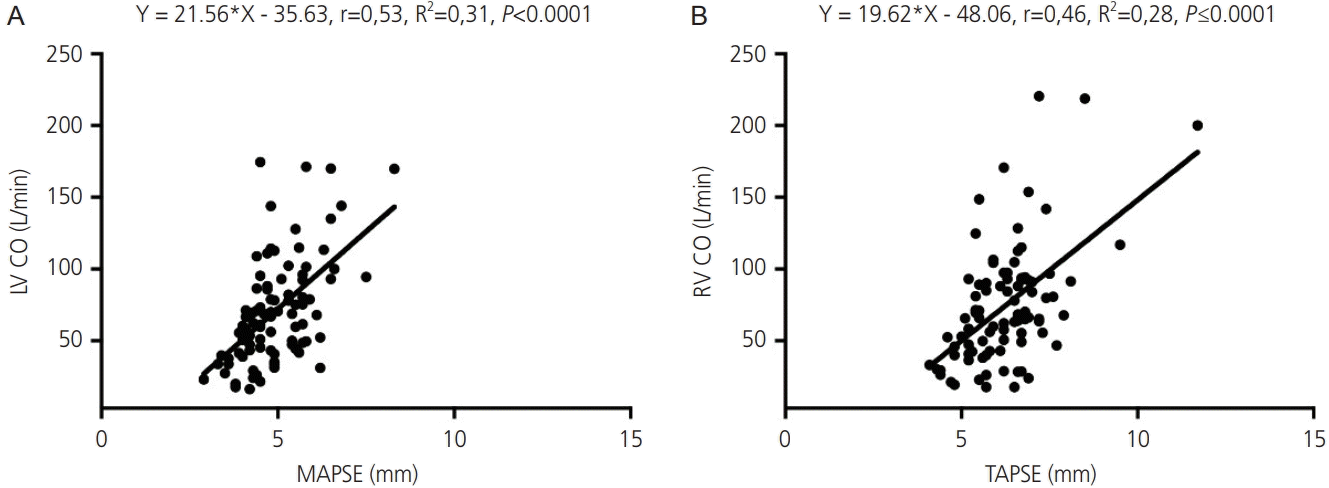
16. Cruz-Lemini M, Crispi F, Valenzuela-Alcaraz B, Figueras F, Sitges M, Gómez O, et al. Value of annular M-mode displacement vs tissue Doppler velocities to assess cardiac function in intrauterine growth restriction. Ultrasound Obstet Gynecol. 2013; 42:175–81.
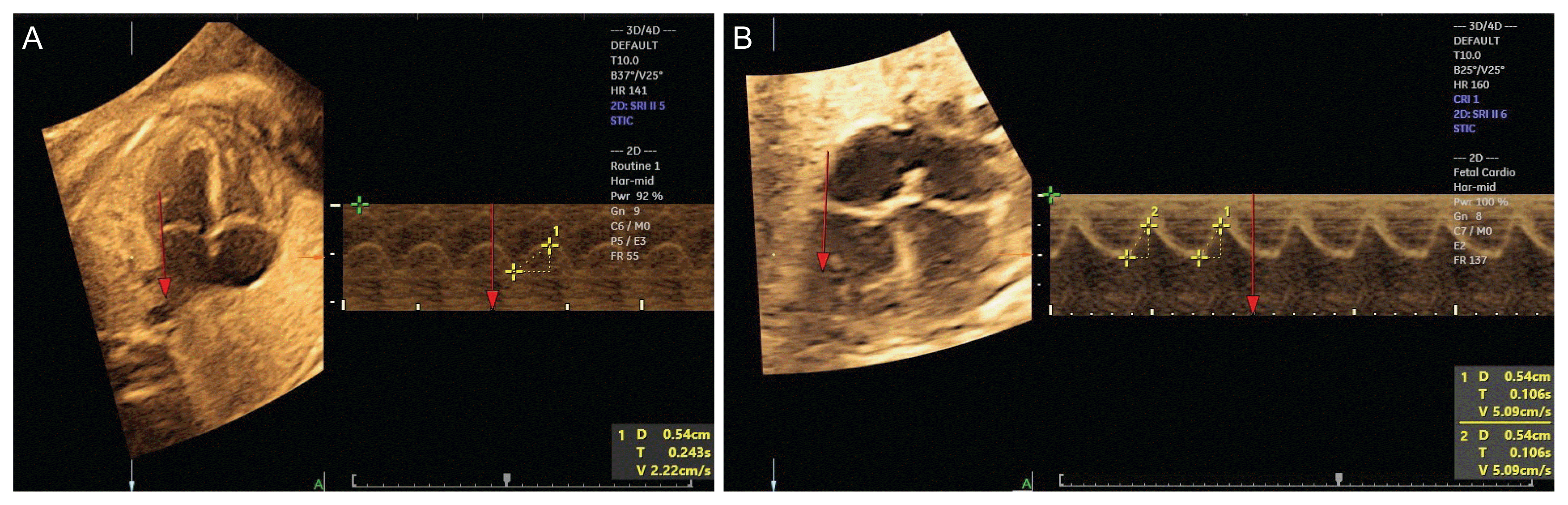
17. Messing B, Gilboa Y, Lipschuetz M, Valsky DV, Cohen SM, Yagel S. Fetal tricuspid annular plane systolic excursion (f-TAPSE): evaluation of fetal right heart systolic function with conventional M-mode ultrasound and spatiotemporal image correlation (STIC) M-mode. Ultrasound Obstet Gynecol. 2013; 42:182–8.

18. Tedesco GD, de Souza Bezerra M, Barros FS, Martins WP, Nardozza LM, Mattar R, et al. Fetal heart function by tricuspid annular plane systolic excursion and ventricular shortening fraction using STIC M-mode: reference ranges and validation. Am J Perinatol. 2017; 34:1354–61.

19. Gonçalves LF, Lee W, Espinoza J, Romero R. Examination of the fetal heart by four-dimensional (4D) ultrasound with spatio-temporal image correlation (STIC). Ultrasound Obstet Gynecol. 2006; 27:336–48.
20. Mao YK, Zhao BW, Wang B. Z-score reference ranges for angular M-mode displacement at 22–40 weeks’ gestation. Fetal Diagn Ther. 2017; 41:115–26.

21. Carvalho JS, O’Sullivan C, Shinebourne EA, Henein MY. Right and left ventricular long-axis function in the fetus using angular M-mode. Ultrasound Obstet Gynecol. 2001; 18:619–22.

22. Germanakis I, Pepes S, Sifakis S, Gardiner H. Fetal longitudinal myocardial function assessment by anatomic M-mode. Fetal Diagn Ther. 2012; 32:65–71.

23. Lee-Tannock A, Hay K, Gooi A, Kumar S. Longitudinal reference ranges for tricuspid annular plane systolic excursion and mitral annular plane systolic excursion in normally grown fetuses. J Ultrasound Med. 2020; 39:929–37.

24. Godfrey ME, Messing B, Valsky DV, Cohen SM, Yagel S. Fetal cardiac function: M-mode and 4D spatiotemporal image correlation. Fetal Diagn Ther. 2012; 32:17–21.

25. Schoonderwaldt EM, Groenenberg IA, Hop WC, Wladimiroff JW, Steegers EA. Reproducibility of echocardiographic measurements of human fetal left ventricular volumes and ejection fractions using four-dimensional ultrasound with the spatio-temporal image correlation modality. Eur J Obstet Gynecol Reprod Biol. 2012; 160:22–9.

26. DeKoninck P, Steenhaut P, Van Mieghem T, Mhallem M, Richter J, Bernard P, et al. Comparison of Doppler-based and three-dimensional methods for fetal cardiac output measurement. Fetal Diagn Ther. 2012; 32:72–8.

27. Simioni C, Nardozza LM, Araujo E Júnior, Rolo LC, Zamith M, Caetano AC, et al. Heart stroke volume, cardiac output, and ejection fraction in 265 normal fetus in the second half of gestation assessed by 4D ultrasound using spatio-temporal image correlation. J Matern Fetal Neonatal Med. 2011; 24:1159–67.

28. Bravo-Valenzuela NJ, Peixoto AB, Mattar R, Melo JF Júnior, da Silva Pares DB, Araujo E Júnior. Fetal cardiac function and ventricular volumes determined by three-dimensional ultrasound using STIC and VOCAL methods in fetuses from pre-gestational diabetic women. Pediatr Cardiol. 2020; 41:1125–34.

29. Hamill N, Yeo L, Romero R, Hassan SS, Myers SA, Mittal P, et al. Fetal cardiac ventricular volume, cardiac output, and ejection fraction determined with 4-dimensional ultrasound using spatiotemporal image correlation and virtual organ computer-aided analysis. Am J Obstet Gynecol. 2011; 205:76.e1–10.

30. Peixoto AB, Bravo-Valenzuela NJ, Martins WP, Tonni G, Mattar R, Moron AF, et al. Reference ranges for the fetal mitral, tricuspid, and interventricular septum annular plane systolic excursions (mitral annular plane systolic excursion, tricuspid annular plane systolic excursion, and septum annular plane systolic excursion) between 20 and 36 + 6 weeks of gestation. J Perinat Med. 2020; 48:601–8.
31. Atiq M, Ikram A, Hussain BM, Saleem B. Assessment of cardiac function in fetuses of gestational diabetic mothers during the second trimester. Pediatr Cardiol. 2017; 38:941–5.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download