Abstract
Objectives
Digital healthcare is expected to play a pivotal role in patient-centered healthcare. It empowers patients by informing, communicating, and motivating them. However, a pragmatic evaluation of the present status of digital healthcare has not been presented; therefore, we aimed to examine the status of digital healthcare in Korea.
Methods
This article discusses digital healthcare, examples of assessment in Korea and other countries, the implications of past examples, and future directions for development.
Results
Over the years, various clinical studies have used clinical evidence to assess the feasibility of digital healthcare. If feasible, it is actually clinically effective. If it is effective, can it be commercialized at an acceptable cost? These questions have been investigated in various evidence-based studies. In addition, great efforts are being made to secure ample evidence to assess various aspects of digital healthcare, such as safety, quality, end-user experience, and equity.
Go to : 
In Korea, long before the term “digital healthcare” was first used, a substantial number of projects using information technology (IT) in providing healthcare services—under varied names such as mobile health, ubiquitous health, and telemedicine—were active. For two decades, digital healthcare services were successful in Korea. The government and industry allocated substantial funds to develop devices, software, and service scenarios, which seemed promising and full of potential. However, none of these services survived, causing discouragement among healthcare providers to attempt using new technology.
Healthcare providers are critical to the success of digital healthcare. Although the expression “digital healthcare” implies high-technology devices and new data, as well as patient-generated health data (PGHD), these elements must be integrated into medicine. Healthcare providers guide patients to perform self-management in safe and effective ways with the help of digital medical devices and platforms.
“Digital healthcare” refers to healthcare services in which data are collected, analyzed, and utilized through the convergence of technologies, including artificial intelligence (AI), big data, the Internet of Things, and cloud computing. According to this definition, there were cases of digital healthcare services, such as, when a fetal heart rate was measured in 1991 [1] and a non-stress test was performed in 1998 [2], remotely. These are examples of remote patient monitoring implemented before the advent of digital healthcare.
Digital healthcare requires a bilateral approach combining medicine and digital technology in a synchronized manner [3]. In addition to increasing the competence of the medical workers, the Korean government must continue to focus on improving individual well-being. Each device, sensor, and element will ultimately have a significant impact on the healthcare industry, and all stakeholders must collaborate to find an efficient way to provide seamless care.
Go to : 
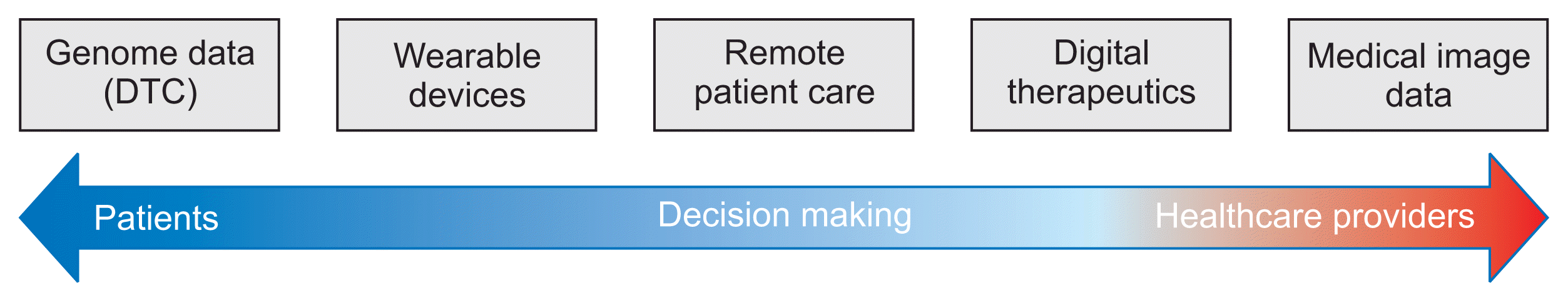
There are numerous cases of digital healthcare services; therefore, systematic classification is required for an effective review. Since there is no universal classification system for digital healthcare, this study proposes a simple classification system for case surveys (Figure 1). The system, illustrated in Figure 1, classifies digital healthcare services according to the service utilization and decision makers (major customers). Considering only the direct-to-consumer (DTC) service, patients’ decision to use the genome information is located to the left of the figure, while healthcare providers’ decision to use medical image information is located at the right-most position. Telemedicine, which presupposes patient–healthcare provider communication, is positioned in the middle. The classification shown in Figure 1 is simplified and is applicable only to this study.
Medical image information is divided into two groups: images that conform to the Digital Imaging and Communications in Medicine (DICOM) standards and medical images such as pathological slides, endoscopic images, and retinographic images.”
IDx-DR by Digital Diagnostics is a device that diagnoses diabetic retinopathy through retinography. After checking the quality of retinographic images based on AI, an oculist determines whether an examination is necessary. Although most AI systems assist decision-making by healthcare providers, IDx-DR was developed to be used without the participation of healthcare providers, becoming the first device to acquire an approval from the US Food and Drug Administration (FDA) [4]. IDx-DR was given the Current Procedure Terminology (CPT) code in the United States, The American Diabetes Association guidelines also mention the potential applicability of the device for screening, indicating that the device has been widely utilized [5]. In addition, significant results from several large-scale clinical studies (with more than 1,000 patients) conducted in many countries have shown that the device has a bright future [6,7].
VUNO received medical device licensing for both bone age reading software and pulmonary nodule reading software, both AI-based software programs. These are representative examples of “software as a medical device” (SaMD) licensed without hardware [8]. VUNO is making continuous efforts to prepare predictive models that are necessary in clinical settings by using vital signs and clinical study information obtained from patients [9]. The data used by VUNO includes general clinical information of Electronic Medical Records (EMRs), emergency medical services, and 12-lead electrocardiogram information. Since acquiring European CE certification for five major AI systems in July 2020, VUNO has extended its business to international markets [10,11].
Lunit gained popularity in South Korea and other countries by sweeping various AI competitions. It entered the medical device market by acquiring a license from the Korean Ministry of Food and Drug Safety in 2018 and the European CE certification in 2019. Lunit’s representative products are Lunit INSIGHT CXR for detecting pulmonary diseases and Lunit INSIGHT MMG for detecting breast cancer. Lunit extends the scope of its business to clinical decision support (CDS) based on clinical information [12]. The efficacy of Lunit’s products has been confirmed by several clinical studies in other countries, and the accuracy of its algorithm for predicting clinical cases has been proven by various medical institutions in South Korea. Additionally, Lunit has strengthened its position in the medical field by continuously publishing its results in major journals [13].
Until recently, digital healthcare was employed as a major method for monitoring patients and for the integration, analysis, and prediction of information. However, digital healthcare has recently been extended to areas of treatment based on powerful mobile devices with analytical capabilities.
Pear Therapeutics, a start-up in the United States, is taking the lead in digital therapeutics. The application “reSET,” developed by the company, was licensed by the FDA in September 2017 making it the first digital therapeutic application [14]. The reSET application is typically used for 90 days with a doctor’s prescription to treat various addictions and dependencies. The reSET-O application, licensed in 2018, is specialized for drug addiction and provides programmed treatment for 84 days. The reSET application assists users with cognitive behavioral therapy: the patients can train themselves to identify triggers linked to addictive substance use by self-monitoring their impulses or changes in thinking. The effect of reSET software on users was proved through randomized controlled trials (RCT) in combination with conventional off-line treatments. Advancing rapidly, Pear Therapeutics developed Somryst, an application for chronic insomnia licensed by the FDA. In July 2020, when corona-virus disease 2019 (COVID-19) was rampant, the company initiated a large-scale clinical study with real patients [15]. As a market leader, Pear Therapeutics is extending its business areas to schizophrenia, epilepsy, and post-traumatic stress disorder [16].
Omada Health provides a software program to prevent and manage diabetes, a widespread problem in the United States, focusing on preventing prediabetic patients from progressing to diabetes and helping them lose weight. The software drew much attention because it was applied in the Diabetes Prevention Program (DPP) promoted by the US Government [17]. Users of the online DPP provided by Omada Health showed a higher program completion rate and more weight loss than users of another DPP based on face-to-face consultation [18]. A 3-year longitudinal study showed that the group of subjects who had successfully completed the program maintained their reduced weight and low glycated hemoglobin levels in the long term [19].
Dexcom is a company that produces a continuous glucose monitoring system (CGMS), which is designed to measure and transmit real-time sugar levels through a device attached to the abdomen, not by blood sampling with a needle or lancet [20]. Remote patient monitoring through such a device facilitates intensive monitoring at home, similar to that in hospitals. However, intensive remote monitoring requires suitable sensors, a mobile system for collecting and transmitting data, an advanced Electronic Health Record (EHR) system for integrating the received data into the existing system, and a service provision system to provide appropriate monitoring and intervention [21].
Teladoc is the oldest and largest telemedicine company in the United States [22]. The average time that patients wait to be connected with a doctor after requesting a consultation is less than 10 minutes [23], and the degree of satisfaction of patients is over 90% [24]. Studies on remote patient monitoring are increasing worldwide, and the devices used for services, diseases, and patient groups subject to these services are also increasing rapidly. Several studies have highlighted the advantages of remote monitoring; however, extensive changes are necessary in the monitoring methods for full-scale implementation of services [25].
The classic wearable device measures physical activity levels. The first commercial wearable activity monitor was known as Fitbit. Subsequently, the activity tracking function was mounted on most wearable devices, such as the Apple Watch and Galaxy Watch, as a basic function. Wearable electrocardiogram (ECG) devices are represented by mobile measurement devices produced by Alivcor. This device measures the ECG when the user holds it with both hands, Additionally, it can analyze the ECG through a smartphone connected via Bluetooth and provide medical recommendations based on data analysis. The initial ECG measurement model received a license from the FDA as early as 2014 for the diagnosis of atrial fibrillation [26]. The Apple Watch received a medical device license from the FDA in 2018 for the ECG measurement function using the stem of the watch [27].
In addition to these examples, a wide variety of sensor services are being released into the mainstream healthcare sector to measure and manage body temperature, sleep, heart rate variability, blood sugar level, oxygen saturation, blood flow, electrical skin stimulation, and anatomical positions. Although, at present, the demand for wearable devices seems low, such devices are expected to play a vital role in healthcare services through detailed service design and technological development.
Genetic information is distinct from other types of digital healthcare information, holding the largest volume of data per case, and the highest potential for growth among digital healthcare services. Whole-genome testing, conducted in laboratories for patients with rare diseases, is raising a massive wave in healthcare services. Although the utilization of genome information is not entirely included in digital healthcare, a DTC service that enables patients to see varied results from genome analysis is considered an area of digital healthcare.
Since its establishment in 2006, 23andMe has provided DTC-type services. Starting in 2013, DTC services with regard to disease incidence risk or sensitivity to drugs were discontinued. In 2017, the FDA licensed the DTC service for the risk analysis of 10 diseases, including Parkinson’s and Alzheimer’s disease. In 2018, the FDA licensed the services for BRCA1 and BRCA2 genes, which are related to the onset of breast cancer [28].
Another establishment, Pathway Genomics provides both hospital-based services and DTC services. These services provided by Pathway Genomics, distinct from 23andMe, are focused on diet and exercise, helping customers customize their health management through genome analysis. Pathway Genomics focuses on health rather than disease.
Go to : 
The Global Digital Health Partnership (GDHP) [29] is a collaboration between various countries and the World Health Organization to share their policies for digital healthcare. The GDHP evaluates evidence of benefits realization of digital healthcare services and aims to strengthen the standardization of international benefit management. In the evidence and evaluation, the GDHP guide presents the following seven categories for measuring the benefits of digital healthcare.
Digital health safety includes issues related to improvements or threats to patient safety associated with the use of digital healthcare services, including digital healthcare errors, adverse events, and privacy.
Digital health quality includes the quality of healthcare services associated with the use of digital healthcare services. This category includes the technical aspects of digital healthcare, as well as the improvement of the medical process to improve quality.
Digital health efficacy refers to the improvement of health status indicators related to digital healthcare services, aimed at the measurement of clinical items for monitoring the effectiveness of digital healthcare, including guidelines for clinicians based on the results.
Digital health end-user experience deals with the experiences of end-users using digital healthcare technology or services. Digital healthcare end-users include patients, consumers, digital healthcare service developers, and policymakers.
This category describes various items that require investment to achieve the best results from digital healthcare, focusing on the relationship between resource inputs, such as labor, capital, and equipment, and the final health state evaluation. This category describes various methods to increase the efficiency of digital healthcare services, reduce unnecessary expenses, and improve productivity.
This category relates to the use of digital healthcare services for purposes that are beneficial to health management in local communities. The goal is to improve health management for particular disease groups or local communities with large populations. The benefits in this category may be evaluated in terms of various measures, such as increased life expectancy and improved emergency systems.
This category is related to healthcare equity, which means that everyone should have a fair opportunity, without unfair or remediable differences, in the distribution of healthcare services; All individuals must be at an equal advantage to access digital healthcare services.
Go to : 
Implementing digital healthcare services is not an easy feat for primary care physicians. A digital health coordinating center (DHCC) is needed as a local hub to support digital healthcare services provided by primary care physicians [30–32].
When a physician prescribes digital healthcare or digital therapeutics at a clinic, the DHCC can educate the patient about the correct way of using digital healthcare services as well as taking relevant precautions. Users are known to visit the DHCC only at the first use or at the first session, visiting the primary clinics at other times. The patient data extracted and saved through the digital healthcare services are transmitted to not only patients but also the DHCC, where medical staff (or coordinators) can monitor the measured data and provide feedback to patients or transmit summaries regarding conditions of individual patients to their primary care physicians. This allows primary care physicians to concentrate on examining patients in their clinics, and use the summaries of various measured data obtained from the hospitals and transmitted by the DHCC. This facility can reduce the burden on primary care physicians in terms of time, cost, and labor, and strengthen the expertise of DHCC.
Currently, since digital healthcare services are provided free of cost, the burden on medical staff and consumers is increasing. In medical centers, the initial training for digital healthcare users requires a considerable amount of time [33]. Nevertheless, owing to insufficient incentives, digital healthcare has not been activated. It is necessary to provide medical staff with incentives for digital healthcare to uplift the main areas of medical services. In addition, it is necessary to reorganize the laws on information protection and responsibility.
Go to : 
The realization of digital healthcare services requires medical records from hospitals, smartphones to measure and collect appropriate data, and a platform that can integrate and accumulate information from various wearable devices, personal genome information, digital phenotypes, and PGHD. A platform called the personal health record (PHR) supports these functions. South Korea has a PHR that is fragmented for individual institutions or purposes; however, it lacks a PHR product that integrates PGHD and shows all the medical and healthcare information of an individual. Since there is no PHR that can satisfy the needs of both individuals and healthcare providers, more investment and research should be implemented in this field. The collected data may be used as medical big data, which can support advances in healthcare by providing new medical and healthcare evidence. Furthermore, the data may be applied as essential data for the development of medical AI.
Go to : 
Notes
Conflict of interest
In Ho Kwon is a member of the Editorial Board of Healthcare Informatics Research; however, he did not involve in the peer review evaluation and decision process of this article. Otherwise, no potential conflicts of interest relevant to this article are reported.
Go to : 
Acknowledgments
This research was supported by the Ministry of Health and Welfare of the Republic of Korea.
Go to : 
References
1. Park MI, Yoo JB. Clinical application of tape-recorder and telemetry system for analysis of fetal heart rate. Korean J Obstet Gynecol. 1991; 34:915–26.
2. Park MI, Hwang YY, Chung SR, Lee JA, Park JI, Koo MK. Fetal heart rate telemetry system for monitoring of high risk pregnancies. Korean J Perinatol. 1998; 9:159–64.
3. Kim HS. Decision-making in artificial intelligence: is it always correct? J Korean Med Sci. 2020; 35:e1.

4. Digital Diagnostics Inc. History of digital diagnostics [Internet]. Coralville (IA): Digital Diagnostics Inc;c2020. [cited at 2021 Mar 30]. Available from: https://dxs.ai/about/history/
.
5. Digital Diagnostics Inc. New CPT code for automated point-of-care retinal imaging [Internet]. Coralville (IA): Digital Diagnostics Inc;c2020. [cited at 2021 Mar 30]. Available from: https://dxs.ai/newsroom/new-cpt-code-for-automated-point-of-care-retinal-imaging/
.
6. Shah A, Clarida W, Amelon R, Hernaez-Ortega MC, Navea A, Morales-Olivas J, et al. Validation of automated screening for referable diabetic retinopathy with an autonomous diagnostic artificial intelligence system in a Spanish population. J Diabetes Sci Technol. 2020. Mar. 16. [Epub]. http://doi.org/10.1177/1932296820906212
.

7. Abramoff MD, Lavin PT, Birch M, Shah N, Folk JC. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. NPJ Digit Med. 2018; 1:39.

8. Jang JW. VUNO: Korea’s first ‘AI-based Diagnostic Assistant Medical Device’ license [Internet]. Seoul, Korea: Biospectator;c2018. [cited at 2021 Mar 30]. Available from: http://www.biospectator.com/view/news_view.php?varAtcId=5485
.
9. Kwon JM, Lee Y, Lee Y, Lee S, Park J. An algorithm based on deep learning for predicting in-hospital cardiac arrest. J Am Heart Assoc. 2018; 7:e008678.

10. Kwon JM, Lee SY, Jeon KH, Lee Y, Kim KH, Park J, et al. Deep learning-based algorithm for detecting aortic stenosis using electrocardiography. J Am Heart Assoc. 2020; 9:e014717.

11. Kang DY, Cho KJ, Kwon O, Kwon JM, Jeon KH, Park H, et al. Artificial intelligence algorithm to predict the need for critical care in prehospital emergency medical services. Scand J Trauma Resusc Emerg Med. 2020; 28:17.

12. Park S, Ahn CH, Jung G, Lee S, Paeng K, Shin J, et al. Deep learning-based predictive biomarker for immune checkpoint inhibitor response in metastatic non-small cell lung cancer. J Clin Oncol. 2019; 37(15_suppl):9094.

13. Lunit Inc. Medical Publications [Internet]. Seoul, Korea: Lunit;c2020. [cited at 2020 Oct 31]. Available from: https://www.lunit.io/ko/evidence/ai-research
.
14. Pear Therapeutics Inc. Pear obtains FDA clearance of the first prescription digital therapeutic to treat disease [Internet]. Boston (MA): Pear Therapeutics Inc;2017. [cited at 2021 Mar 30]. Available from: https://peartherapeutics.com/fda-obtains-fda-clearance-first-prescription-digital-therapeutic-treat-disease/
.
15. Pear Therapeutics Inc. Pear Therapeutics announces first participant enrolled in virtual real-world study of adults with chronic insomnia [Internet]. Boston (MA): Pear Therapeutics Inc;c2020. [cited at 2021 Mar 30]. Available from: https://peartherapeutics.com/pear-therapeutics-announces-first-participant-enrolled-in-virtual-real-world-study-of-adults-with-chronic-insomnia/
.
16. Pear Therapeutics Inc. Product pipeline [Internet]. Boston (MA): Pear Therapeutics Inc;c2021. [cited at 2021 Mar 30]. Available from: https://peartherapeutics.com/science/product-pipeline/
.
17. National Institute of Diabetes and Digestive and Kidney Diseases. Diabetes Prevention Program (DPP) [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases;c2020. [cited at 2021 Mar 30]. Available from: https://www.niddk.nih.gov/about-niddk/research-areas/diabetes/diabetes-prevention-program-DPP
.
18. Moin T, Damschroder LJ, AuYoung M, Maciejewski ML, Havens K, Ertl K, et al. Results from a trial of an online diabetes prevention program intervention. Am J Prev Med. 2018; 55:583–91.

19. Sepah SC, Jiang L, Ellis RJ, McDermott K, Peters AL. Engagement and outcomes in a digital Diabetes Prevention Program: 3-year update. BMJ Open Diabetes Res Care. 2017; 5:e000422.

20. Jung SW. Continuous Glucose Monitoring System (CGMS) covered by health insurance [Internet]. Seoul, Korea: Newspim;2020. [cited at 2021 Mar 30]. Available from: https://www.newspim.com/news/view/20200114001060
.
21. Kumar RB, Goren ND, Stark DE, Wall DP, Longhurst CA. Automated integration of continuous glucose monitor data in the electronic health record using consumer technology. J Am Med Inform Assoc. 2016; 23:532–7.

22. US Securities and Exchange Commission. Amendment No. 3 to Form S-1 Registration Statement under the Securities Act of 1933 [Internet]. Washington (DC): US Securities and Exchange Commission;2015. [cited at 2021 Mar 30]. Available from: https://www.sec.gov/Archives/edgar/data/1477449/000104746915005538/a2225135zs-1a.htm
.
23. Teladoc Health Inc. Teladoc blazes a trail in the emerging virtual telehealth services industry [Internet]. Purchase (NY): Teladoc Health Inc;2016. [cited at 2021 Mar 30]. Available from: https://ir.teladochealth.com/news-and-events/investor-news/press-release-details/2016/Teladoc-blazes-a-trail-in-the-emerging-virtual-telehealth-services-industry/default.aspx
.
24. Versel N. Seven things we learned from the Teladoc IPO registration [Internet]. New York (NY): MedCityNews;c2015. [cited at 2021 Mar 30]. Available from: https://medcitynews.com/2015/05/seven-things-we-learned-from-the-teladoc-ipo-registration/
.
25. Noah B, Keller MS, Mosadeghi S, Stein L, Johl S, Delshad S, et al. Impact of remote patient monitoring on clinical outcomes: an updated meta-analysis of randomized controlled trials. NPJ Digit Med. 2018; 1:20172.

26. AliveCor Inc. AliveCor receives first FDA clearance to detect a serious heart condition in an ECG on a mobile device [Internet]. Mountain View (CA): Alive-Cor Inc;2014. [cited at 2021 Mar 30]. Available from: https://www.alivecor.com/press/press_release/alivecor-receives-first-fda-clearance-to-detect-a-serious-heart-condition-in-an-ecg-on-a-mobile-device/
.
27. Choi YS. A study on the ECG measurement of Apple Watch 4 [Internet]. Seoul, Korea: YoonSup Choi’s Healthcare Innovation;c2018. [cited at 2021 Mar 30]. Available from: https://www.yoonsupchoi.com/2018/09/21/apple-watch4-ecg/
.
28. Begley S. FDA approves first direct-to-consumer test for breast cancer risk [Internet]. Boston (MA): STAT;2018. [cited at 2021 Mar 30]. Available from: https://www.stat-news.com/2018/03/06/fda-approves-test-breast-cancer/
.
29. Global Digital Health Partnership. Evidence and evaluation [Internet]. New Delhi, India: Global Digital Health Partnership;c2020. [cited at 2021 Mar 30]. Available from: https://gdhp.nhp.gov.in/home/EvidenceEvaluation
.
30. Kim HS, Shin JA, Chang JS, Cho JH, Son HY, Yoon KH. Continuous glucose monitoring: current clinical use. Diabetes Metab Res Rev. 2012; 28(Suppl 2):73–8.

31. Kim HS, Lee KH, Kim H, Kim JH. Using mobile phones in healthcare management for the elderly. Maturitas. 2014; 79:381–8.

32. Kim HS, Sun C, Yang SJ, Sun L, Li F, Choi IY, et al. Randomized, open-label, parallel group study to evaluate the effect of Internet-based glucose management system on subjects with diabetes in China. Telemed J E Health. 2016; 22:666–74.

33. Jung SH, Kim JW, Kang IK, Park CY, Kim YS, Woo JT. Continuous glucose monitoring is needed to detect unrecognized hypoglycemic event in diabetic patients with stroke. J Korean Diabetes. 2002; 3:140–51.
Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download