INTRODUCTION
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is characterised by necrotising vasculitis that affects small vessels, including arterioles, venules, and capillaries. The 2012 Chapel Hill Consensus Conference Nomenclature of the Vasculitides (the 2012 CHCC definitions) and the 2007 European Medicines Agency algorithm (the 2007 EMA algorithm) indicated that AAV consists of three subtypes: microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA), and eosinophilic granulomatosis with polyangiitis (EGPA) [
1,
2]. Although various immune mechanisms are involved in its pathogenesis, the alternative complement pathway is central to the acceleration and maintenance of the vicious inflammatory cycle of AAV [
3,
4]. Therefore, with the progression of inflammation, the consumption of complement components may be theoretically increased, resulting in hypocomplementemia.
The total haemolytic complement activity (CH50) assay is a widely used assay to evaluate abnormalities of all complement components (C1 to C9). CH50 can be reduced owing to congenital complement deficiencies, increased complement consumption, or decreased complement synthesis [
5]. In the pathogenesis of AAV, the alternative complement system is activated and generates C5a, which attracts and prime neutrophils [
6]. Considering that CH50 assesses the overall activity of the whole complement system, and that hypocomplementemia has been reported in patients with vasculitis and associated with the patient prognosis [
7-
10], we aimed to investigate whether CH50 at diagnosis reflects the baseline activity and predicts disease outcomes during follow-up in immunosuppressive drug-naïve AAV patients in this study.
MATERIALS AND METHODS
Patients
We included and reviewed the medical records of 101 immunosuppressive drug-naïve patients with AAV who were evaluated at the tertiary medical center, between October 2000 and December 2019. All patients were classified or reclassified to have MPA, GPA, or EGPA based on the 2007 EMA algorithm and the 2012 CHCC definition [
1,
2]. All patients had well-documented medical records to obtain clinical and laboratory results and to calculate the Birmingham Vasculitis Activity Score (BVAS) and the Five Factor Score (FFS) at the time of diagnosis [
11,
12]. Additionally, all patient records had information for both tests for ANCA and complements, such as CH50, C3, and C4 [
13,
14], and all patients had been followed up for at least 3 months. None of the patients had any serious medical condition, such as malignancies and serious infections, at the time of diagnosis, and they had never received any immunosuppressive drug until diagnosis. This study was approved by the Institutional Review Board of Severance Hospital (4-2017-0673). The need for written informed consent was waived owing to the retrospective nature of this study.
Clinical data at diagnosis and during follow-up
Regarding variables at diagnosis, information on age and sex was collected as demographic data. Clinical data, such as those on AAV subtypes, and those pertaining to disease activity, including the BVAS and FFS, were collected. Laboratory data, including those of white blood cell and platelet counts, haemoglobin, fasting glucose, blood urea nitrogen (BUN), creatinine, total serum protein, serum albumin, alkaline phosphatase (ALP), aspartate aminotransferase, alanine aminotransferase, total bilirubin, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP), were obtained. In addition, ANCA and CH50 data were collected. Data related to poor prognosis outcomes, including all-cause mortality, relapse, and end-stage renal disease (ESRD), were evaluated. Relapse was defined as recurrence or new onset disease requiring an increase in glucocorticoids and switching the immunosuppressant due to increased disease activity. ESRD was defined as a medical condition requiring renal replacement therapy for >3 months. The follow-up duration was defined as the interval between the date of the diagnosis of AAV and date of the last visit for living patients. For deceased patients, it was defined as the interval between the date of diagnosis of AAV and the date of death, whereas for patients who had other poor outcomes, it was defined as the interval starting from the date of diagnosis of AAV until the date of occurrence of the first poor outcome.
Classification of variables: Tertiles based on the BVAS and two groups based on CH50
Since there is no clear BVAS criterion to define high disease activity, we divided the baseline BVAS into tertiles: high activity, intermediate activity, and low activity. We defined baseline high disease activity as a BVAS ≥13, which is the highest tertile in our data. Furthermore, we categorised the patients with AAV into two groups based on the calculated optimal cut-off of CH50 for high baseline activity: patients with CH50 levels below the optimal cut-off were classified into the low-CH50 group, and those with CH50 levels equal to or above the cut-off were classified into the high-CH50 group.
Statistical analyses
All statistical analyses were conducted using SPSS software (version 23 for Windows; IBM Corp., Armonk, NY, USA). Continuous variables were expressed as the median and interquartile range, whereas categorical variables were demonstrated as frequencies and percentages. The correlation coefficient was obtained using Pearson correlation analysis. The optimal cut-off was extrapolated by calculating the receiver operator characteristic curve and selecting the maximised sum of sensitivity and specificity. The relative risk (RR) was analysed using contingency tables and the chi-square test. The standardised correlation coefficient was assessed by multivariable linear regression analysis using variables showing significant differences in univariable analysis. The cumulative survival rates between the groups were compared using Kaplan–Meier survival analysis with the log-rank test. p-values<0.05 were considered statistically significant.
DISCUSSION
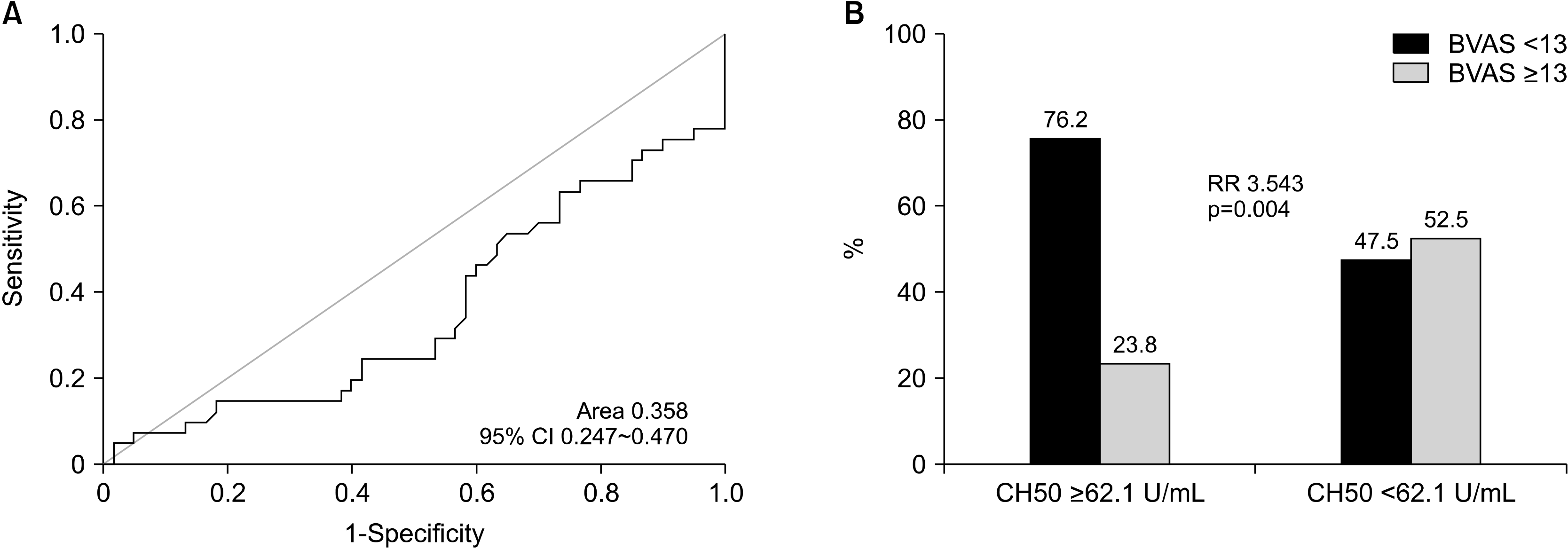
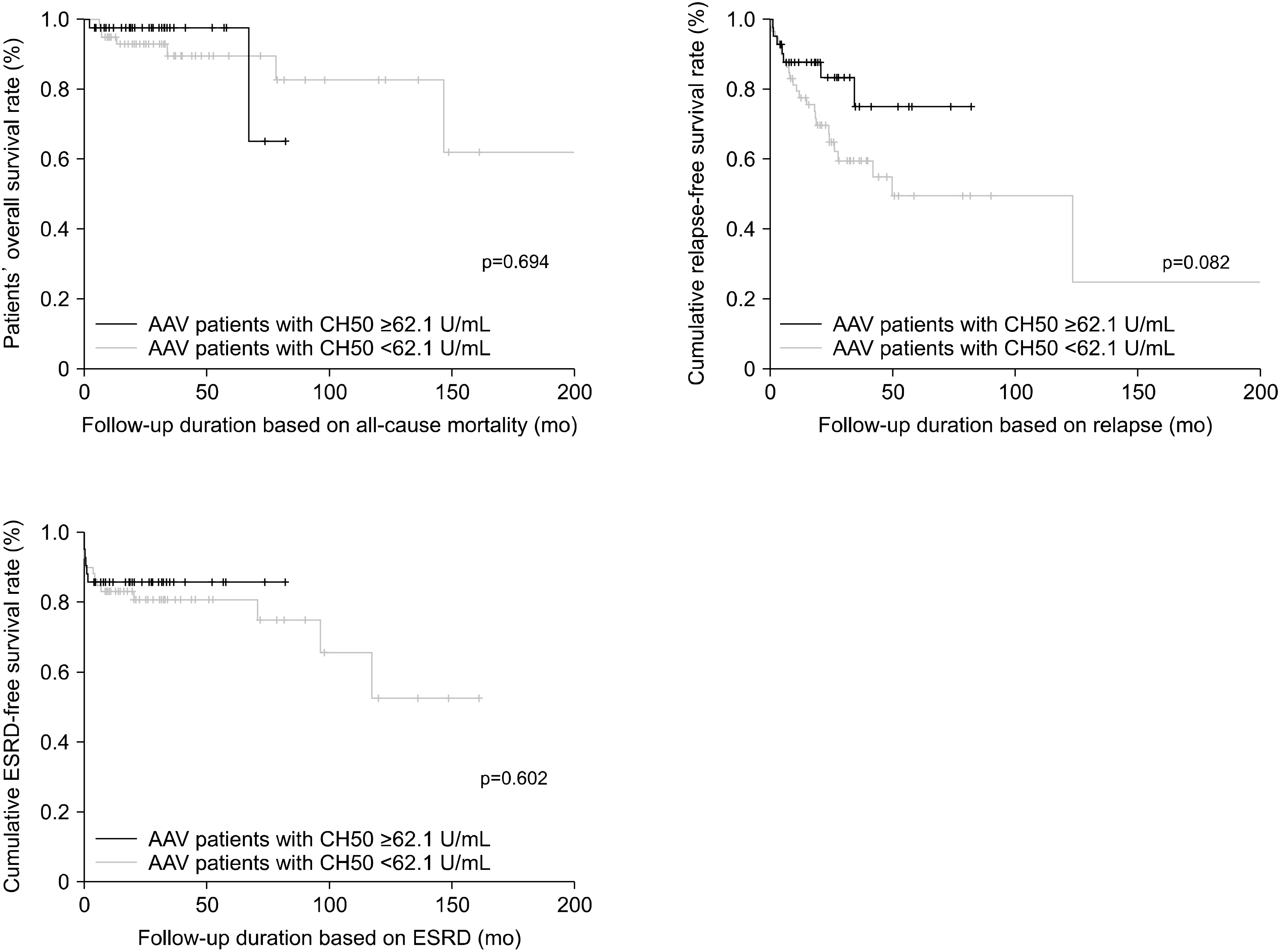
In this study, we investigated whether CH50 levels at diagnosis could reflect the baseline activity and predict the prognosis of AAV. At the diagnosis of AAV, CH50 was inversely correlated with the BVAS, and based on our data, the suggested cut-off CH50 level to predict severe disease of AAV was 62.1 U/mL. Summarising the results of this study, the following conclusions were drawn: the low CH50 level at diagnosis was associated with severe disease of AAV; however, it did not predict the occurrence of poor outcomes of AAV.
Among C3 and C4, only C4 positively correlated with CH50, which primarily reflected the activation of the classical complement pathway. At the beginning of this study, C3 was also expected to be well-correlated with CH50 because the alternative complement pathway is known to be mainly involved in the pathophysiology of AAV compared with the classical complement pathway. However, no correlation was found between CH50 and C3. Therefore, to validate this finding, a method of interpreting ‘which complement pathway is activated’ was adopted, based on the patterns of increases and decreases in CH50, C3, and C4 levels. In this method, when CH50 and C4 levels were reduced, regardless of C3 levels, we found that the classical complement pathway was predominantly involved. Concurrently, when CH50 and C3 levels were reduced and the C4 level was normal, we considered that the alternative complement pathway was more frequently involved [
15]. In the present study, 6 out of 101 patients had a CH50 level below the normal reference range according to the haemolysis method, and all these patients exhibited a high baseline activity of AAV based on the BVAS. Furthermore, three of the six patients with a CH50 level below the normal reference range exhibited low C3 or C4. One patient showed low C3, which indicated alternative complement pathway activation. However, another patient demonstrated low C4, and the third patient exhibited both low C3 and C4, which suggested a classical complement pathway activation. With these results, we could not conclude which complement pathway was predominantly contributing to the high baseline activity of AAV.
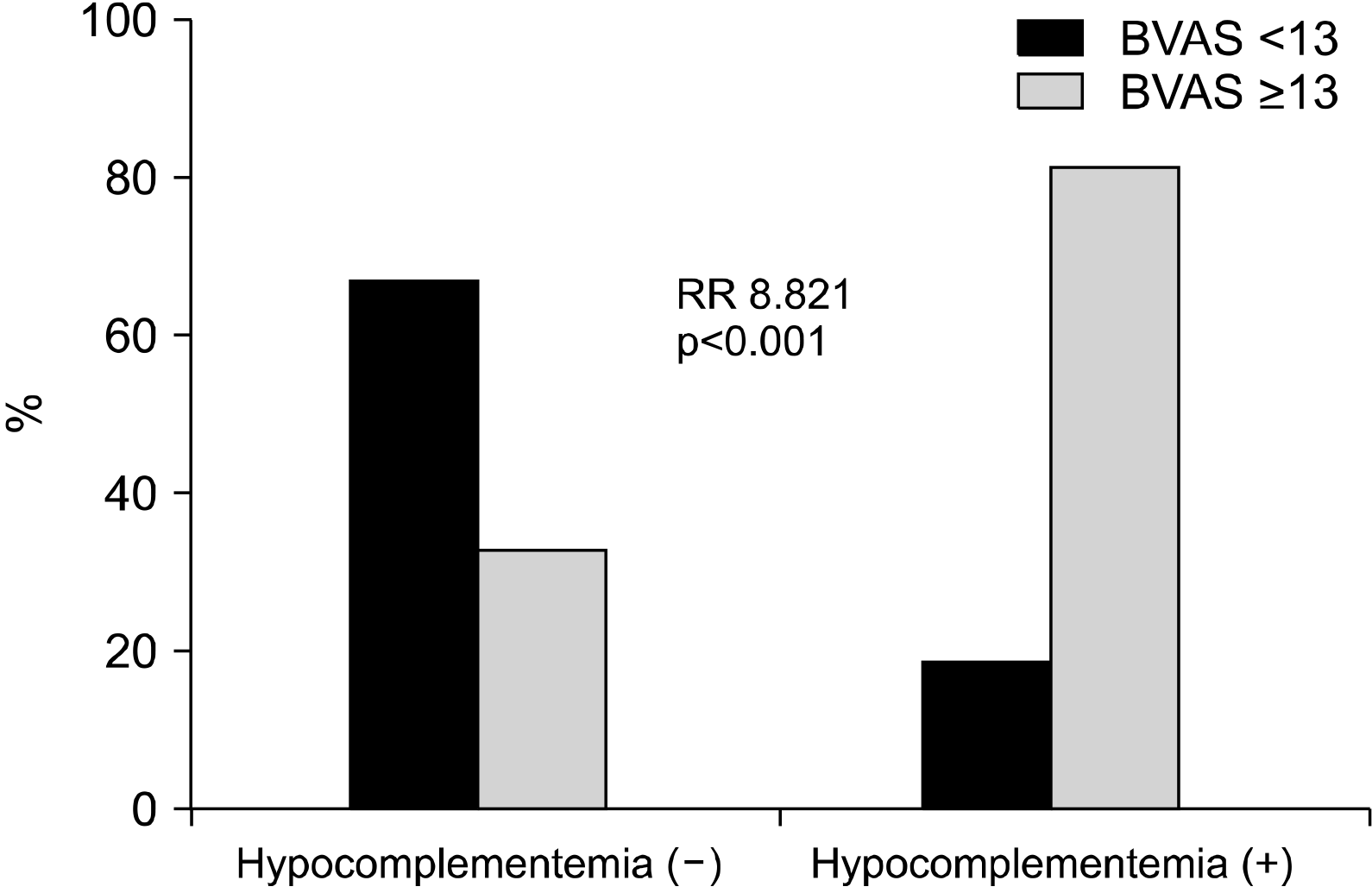
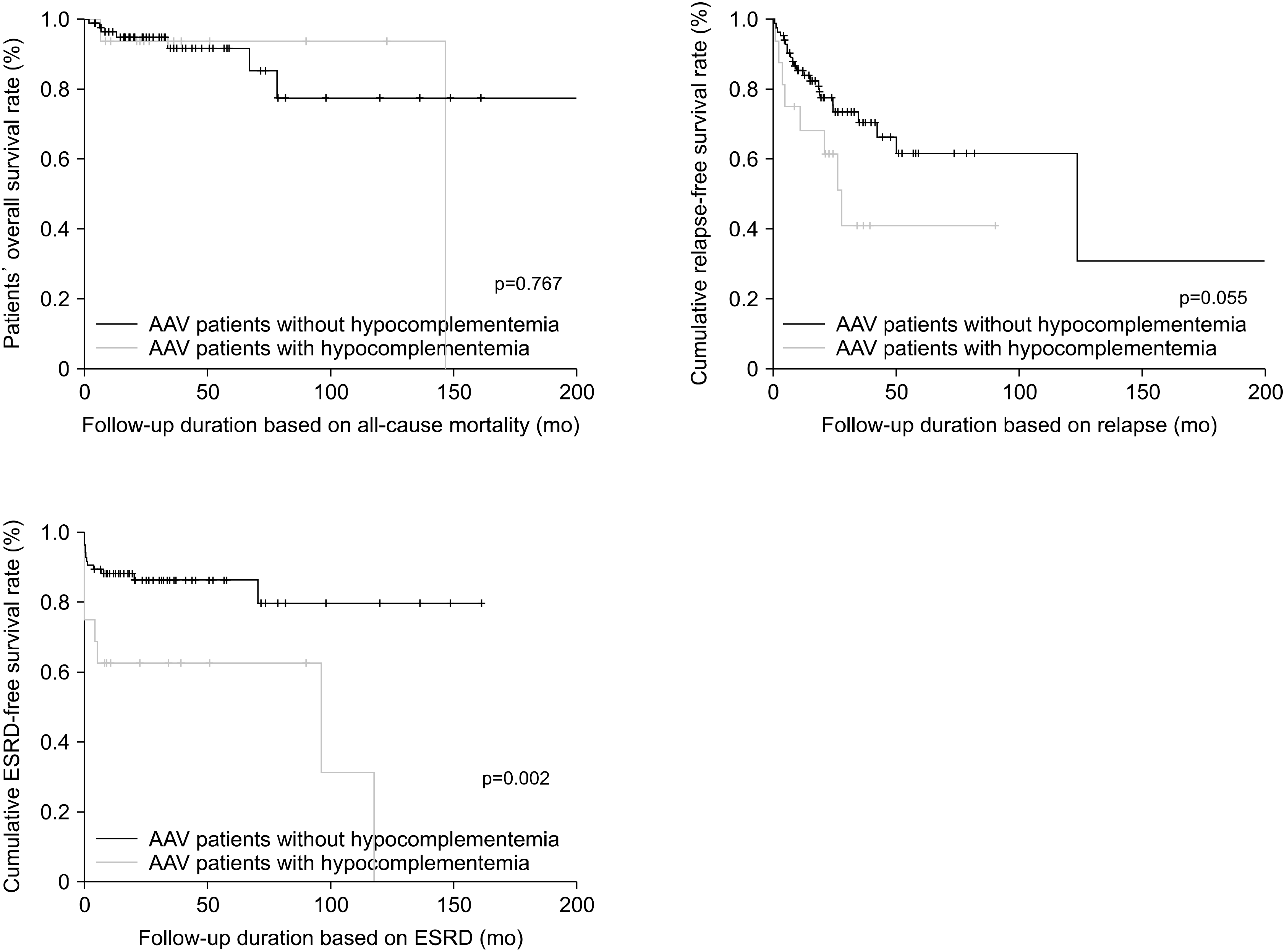
Fukui et al. [
9] reported that hypocomplementemia, which was defined as a low CH50, C3, or C4 level, was associated with death and organ damage in patients with AAV. In this study, we investigated whether hypocomplementemia could reflect the high baseline activity of AAV and predict poor outcomes. Our data showed that AAV patients with hypocomplementemia showed a significantly higher risk of high baseline activity (based on the BVAS, BVAS ≥13) and significantly lower cumulative ESRD-free survival than those without hypocomplementemia. However, unlike that in the previous study [
9], no significant differences were observed in both the cumulative patients’ overall and relapse-free survival rates between the groups. Further studies are needed to analyze these discrepancies and conclude an association between hypocomplementemia and disease activity and poor outcomes of AAV. Moreover, we examined whether low C3 or low C4 could reflect the baseline activity of AAV and predict ESRD occurrence (data not presented). We found that AAV patients with low C3 exhibited a significantly higher risk of high baseline activity of AAV, based on the BVAS, than those with normal C3 levels (RR, 5.129; 95% CI, 1.291∼20.382). Furthermore, a low C3 value at diagnosis had the predictive potential for ESRD occurrence during follow-up, compared with a normal C3 (p=0.001). Conversely, a low C4 value at diagnosis could neither reflect a high baseline activity of AAV based on the BVAS nor predict the occurrence of ESRD during follow-up in AAV patients (p=0.069 and 0.061, respectively). Consequently, we conclude that low CH50 and low C3 among patients with hypocomplementemia reflect the baseline activity, whereas only low C3 predicts ESRD occurrence during follow-up.
In this study, we used a CH50 value of 61.2 U/mL, rather than the reference range, as the optimal cut-off for the high baseline activity of AAV. In the univariable analysis, at diagnosis, both CH50 <62.1 U/mL (OR, 3.543; 95% CI, 1.477∼8.497) and low C3 (OR, 5.129; 95% CI ,1.291∼20.382) were significantly associated with the high baseline activity of AAV based on the BVAS. However, in the multivariable analysis with two variables, CH50 <62.1 U/mL (OR, 3.320; 95% CI, 1.327∼8.306) was significantly associated with high baseline activity of AAV; however, low C3 (OR, 3.389; 95% CI, 0.812∼12.147) was not. Therefore, CH50 below the optimal cut-off is more efficient than low C3, reflecting the high baseline activity of AAV based on the BVAS; however, for predicting poor renal outcomes, low C3 at diagnosis is better than CH50 below the optimal cut-off at diagnosis.
A notable strength of this study is that we provided a novel cut-off value for CH50, which was correlated with baseline disease activity. Furthermore, our patient population was immunosuppressant-naïve and diagnosed at a single centre, which can minimise the confounding factor. However, our study had the following limitations. First, the number of study subjects was not large enough to generalise and apply the results of our study to all patients with AAV. Second, because we do not have separate validation group, the cut-off value of CH50 as 61.2 U/mL should be validated in other AAV cohorts in future studies. In addition, it was conducted as a retrospective study. Hence, alternative complement pathway activation (AH50) and Factor B could not be evaluated in this study. Inclusion of AH50 and Factor B might have enabled a more accurate interpretation of the CH50, C3, and C4 patterns [
5,
15].




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download