Abstract
Objectives
In this study, I aimed to evaluate the inhibitory effect of bile acids on the inflammatory response and osteoclastogenesis in RAW 264.7 cells activated through lipopolysaccharide (LPS) and receptor activator of nuclear factor-κB ligand (RANKL) of the periodontal pathogen Porphyromonas gingivalis.
Methods
Myelomonocytic RAW 264.7 cells were activated through P. gingivalis LPS to induce inflammatory response, and were treated with three bile acids, including taurodeoxycholate, taurocholate, and glycocholate at different concentrations. The cytotoxicity of bile acids was assessed through the MTT assay. To evaluate the inhibitory effect of bile acids on inflammatory response, the induction levels of the pro-inflammatory cytokines, such as interleukin (IL)-6 and tumor necrosis factor (TNF)-α were measured using ELISA 12 h after the treatment. Additionally, after activating the cells with RANKL to promote osteoclastogenesis, we examined whether bile acids suppressed osteoclast differentiation using the tartrate-resistant acid phosphatase staining.
Results
In the cell viability test, taurodeoxycholate and taurocholate did not exhibit any cytotoxic effect on RAW 264.7 cells at concentrations equal to or less than 200 mM, and glycocholate was non-cytotoxic until the maximal concentration (4,000 mM). All the three bile acids exhibited an inhibitory effect on inflammatory response, as the production levels of pro-inflammatory cytokines, including IL-6 and TNF-α, decreased with an increase in the concentration of the three bile acids in a dose-dependent manner. The expression of IL-6 reduced remarkably upon treatment with taurodeoxycholate and glycocholate (P<0.001), while the expression of TNF-α decreased slightly upon treatment with glycocholate (P<0.05). Moreover, only glycocholate at a concentration of 1,000 µM suppressed osteoclast differentiation of RAW 264.7 cells (P<0.001), while taurodeoxycholate and taurocholate did not exhibit an inhibitory effect on osteoclastogenesis.
Periodontitis is involved with oral microorganisms, environmental conditions, and host factors. The host-immune system often responses to microbial or environmental factors to aggregate inflammatory cells and increase the expression of pro-inflammatory cytokine such as interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α, proteolytic enzymes, and bone metabolism-related factors, finally resulting in destruction of periodontal tissue1-3).
Especially, gram-negative anaerobes in biofilms such as Porphyromonas gingivalis and its lipopolysaccharide (LPS) play an important role in the etiopathogenesis of periodontitis. P. gingivalis has a variety of virulence factors and induces the secretion of pro-inflammatory cytokines in periodontal tissue, thereby activating a receptor activator of nuclear factor-κB ligand (RANKL) and osteoclast expression and resulting in alveolar bone destruction1-3). In recent years, peri-implant diseases with similar characteristics to periodontal diseases have also increased, the periodontopathogens closely related to periodontitis such as P. gingivalis and Treponema denticola were also found at high levels in inflamed peri-implant sites4,5).
The periodontal pathogens and its metabolites in periodontal tissue could impact on systemic conditions6-8). Many studies have suggested that periodontal disease and several chronic diseases are correlated through the action of common risk factors. Comprehensively, chronic inflammation can be described as a shared mechanism of linking periodontal diseases including periodontitis and peri-implantitis, and various systemic diseases9-12). Therefore, the control of the inflammatory process is an essential target for the management of both periodontal and systemic diseases.
These days, inflammation has become more important as a critical factor in the pathogenesis of many systemic diseases. Many studies have studied on substances capable of regulating the immune-inflammatory response and the accompanying tissue-destructive metabolism. In particular, the anti-inflammatory effects of well-known natural substances, foods, and substances derived therefrom are evaluated. In dentistry, it is noted whether the anti-inflammatory components can also inhibit periodontal inflammation and breakdown such as bone resorption2,13-15). For example, it was confirmed that milk and dairy products inhibited expression of pro-inflammatory cytokines in gingival fibroblast as well as intestinal inflammation15). The anti-inflammatory action of food-derived antioxidants or commonly used antibiotics also had the impact on inflammatory process induced by P. gingivalis LPS2,13,14).
Bile acids or bile salts as a major component of bile juice emulsify fat to help lipolysis and form an osmotic gradient to promote bile secretion. Glycocholate and taurocholate are the primary bile acids, and secondary bile acids are sometimes produced by gut microbiota16). Bile and related extracts (eg, bear bile) are used as a cholagogue (choleretic) or improvement of liver function in clinical use. In addition, several recent studies reported the potentials of bile acids as a regulator for inflammation. Bile acids can bind to specific receptors to regulate inflammation17), and help inhibiting various inflammatory diseases18-24).
One of bile acids, glycocholate, presented an inhibitory effect on inflammatory activation induced by E. coli LPS. It suppressed the activations of dendritic cells and subsequent T cells by LPS and also reduced the expressions of TNF-α and IL-12p40 in murine model18). In another study, taurocholate also decreased the levels of IL-1β, interferon-γand TNF-α in colon tissues and alleviated inflammatory signs on ulcerative colitis21). Secondary bile acid, taurodeoxycholate was also examined as a therapeutic agent to prevent sepsis and atopic dermatitis. Its intravenous infusion suppressed septic response triggered with LPS and alleviated abnormal inflammatory reactions on cutaneous tissues19,20). Taurodeoxycholate also had anti-inflammatory effect on stomach and promoted the proliferation of intestinal cells and inhibited the apoptosis induced by the activation of NF-κB23,24).
The anti-inflammatory action of bile acids in other tissues can also be involved in the inflammatory mechanism of periodontal tissue. The regulation of inflammation is both locally and universally important as considering the inflammation as a link between periodontitis and systemic diseases. However, no studies have evaluated whether bile acids can control periodontal inflammation and its accompanying tissue-destructive processes. Therefore, it is necessary to investigate the potential of these universal inflammatory regulators exert on process of periodontitis. Therefore, this study aimed to evaluate the inhibitory effect of bile acids on the inflammatory response and osteoclastogenesis in macrophage cell line RAW 264.7 stimulated by periodontopathogens P. gingivalis-LPS and RANKL.
Three bile acids (bile salts) were selected as experimental substances. They are sodium taurodeoxycholate (Sigma, St. Louis, MO, USA), sodium taurocholate (Alfa Aesar, Haverhill, MA; Sigma), and sodium glycocholate (Sigma). P. gingivalis 33277 LPS (Pg-LPS; InvivoGen, San Diego, CA, USA), the macrophage colony-stimulating factor (M-CSF; R&D systems, MN, USA), and recombinant truncated mouse RANKL (R&D systems) were used as cell stimulators.
Murine cell-line RAW 264.7 (ATCC® TIB-71TM; American Type Culture Collection, Manassas, VA, USA; RRID:CVCL_0493) were used. This myelomonocytic cell line can differentiate into macrophages which are immune cells regulating inflammatory response or into osteoclasts depending on surrounding regulatory factors such as M-CSF, RANKL, and physiological environment.
RAW 264.7 cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Sigma) with high glucose (4,500 mg/L), supplemented with 10% fetal bovine serum (FBS; Gibco, Grand Island, NY, USA), penicillin (100 U/ml), and streptomycin (100 mg/ml) (Sigma). They were incubated in 100 mm culture dishes (Corning, Corning, NY, USA) under 37℃, 5% CO2, and humidified conditions.
Adherent cells in culture dishes were collected by a cell scraper after culturing in 1-3 days and seeded in 6, 24, and 96-well plates (Corning and SPL, Pocheon, Korea). The cells were activated by LPS (1 µg/ml) from the P. gingivalis strain to induce inflammatory responses similar to periodontitis, and stimulated by M-CSF (30 ng/mL) and RANKL (100 ng/mL) to promote osteoclastogenesis. Three bile acids were treated at different concentrations (0-4,000 µM) in the absence or presence of Pg-LPS, M-CSF, and RANKL. The treatment and control cells were incubated for different hours according to experiments.
Cell viability or cell cytotoxicity was assessed by MTT-assay. RAW 264.7 cells were seeded at a density of 3×103 cells/well onto 96-well plates and incubated in a 37℃ and 5% CO2 for 24 hours. The wells were treated then with either only culture medium 100 ml (control) or mixture of three bile salts at different concentrations with or without Pg-LPS (1 mg/ml); sodium taurodeoxycholate and sodium taurocholate were treated at the range of 0-1,000 µM (0, 2, 5, 10, 20, 50, 100, 200, 500, and 1,000 µM), and sodium glycocholate at the range of 0-4,000 µM (0, 20, 50, 100, 200, 500, 1,000, 2,000, and 4,000 µM), each. After 24 hours incubating, 20 µl of 5 mg/ml 3-(4,5-Dimethyl -2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT; Alfa Aesar) solution was added in each well. The plates were incubated for 3 hours further for the formation of MTT formazan, which reflects the amounts of living cells. After the removal of the supernatant and adding of 100 µl DMSO solution, the optical density was measured at 570 nm on a microplate reader.
RAW 264.7 cells were cultured at a density of 3×104 cells/well in 6-well plates in a 37℃ and 5% CO2 for 24 hours. The wells were treated then with either culture medium alone (control) or three bile salts at serial concentrations under the stimulation of Pg-LPS (1 µg/ml). According to the results of cell viability test, sodium taurodeoxycholate and sodium taurocholate were treated in the range of 0-100 µM (0, 1, 10, and 100 µM) and sodium glycocholate were done in the range of 0-1,000 µM (0, 1, 10, 100, and 1,000 µM), as the limited concentration without cell toxicity. Culture supernatants were collected after incubating for 12 hours (the treatment time was determined by preliminary experiments done with several time points), and stored at ―80℃.
The levels of pro-inflammatory cytokines IL-6 and TNF-α in the culture supernatants were measured by enzyme-linked immunosorbent assay (ELISA). ELISA kit (Ready-SET-Go; eBioscience Affymetrix, San Diego, CA, USA) and related reagents were used in accordance with the manufacturer’s instructions. After reagent treatments, the optical density of the plates was measured at 450 nm.
2×103 cells/well of RAW 264.7 cells were seeded in 96-well plates and treated with Pg-LPS (1 µg/ml) and/or RANKL (100 ng/mL) combined with M-CSF (30 ng/mL). Three bile salts were added in the range of 0-1,000 µM (0, 1, 10, 100, and 1,000 µM). After 3 days incubating, the culture medium was exchanged by fresh control or reagent-treated ones, and the cells were allowed to differentiate for 1 day more. After total 4 days of culturing, the cells were fixed with 3.7% paraformaldehyde (Alfa Aesar) for 15 minutes and permeabilized by 0.1% Triton X-100 (Sigma) for 1 minute.
Tartrate-resistant acid phosphatase (TRAP) staining was carried out with TRAP kit (Leukocyte Acid Phosphatase kit; Sigma) according to the manufacturer’s instruction. TRAP-positive multinucleated cells (with ≥3 nuclei) were regarded as osteoclast or osteoclast-like cells (OLCs), which were counted under a digital inverted light microscope with the camera (Nikon, Tokyo, Japan).
Results were presented as the mean and standard deviation (S.D). One-way analysis of variance (ANOVA) followed by Tukey test was used in the statistical analyses and P-values <0.05 were considered statistically significant. All statistical analyses and graphics were performed by Graphpad Prism version 5.01 software (Graphpad software Inc., Graphpad San Diego, CA, USA, RRID:SCR_002798).
RAW 264.7 cells were treated with culture medium alone or in combination with bile salts, and then cell viability was measured after 24 hours by MTT-assay. In results, cell viabilities of three bile salts-treated groups were not significantly different compared to that of the control group at most concentrations (Fig. 1). The cell viabilities of sodium taurodeoxycholate and sodium taurocholate were similar to or higher than that of the control group at the concentration of less than 200 µM. However, both compounds presented cytotoxicity to the cells 500 and 1,000 µM (P<0.001) (Fig. 1A, B). Meanwhile, sodium glycocholate presented nontoxic effect up to 4,000 µM, a maximum concentration of the experiment (Fig. 1C).
To evaluate the effect of three bile acids on the inflammatory response, the induction levels of the pro-inflammatory cytokines IL-6 and TNF-α after treatment of the compounds during 12 hours were measured by ELISA. The production of IL-6 and TNF-α increased significantly in the cell supernatants stimulated under Pg-LPS alone compared to the control ones, while the expression of both cytokines tended to be suppressed with the increasing of concentration of three bile salts in a dose-dependent manner (Fig. 2). Notably, the production of IL-6 was strongly inhibited at concentration higher than 100 µM of sodium taurodeoxycholate or 10 mM of sodium glycocholate, each (P<0.001) (Fig. 2A, C). The induction of TNF-α was also suppressed as the concentration of each bile salt increased, however, the significance of the trend was relatively weak compared to that of IL-6. The levels of TNF-α were significantly lowered only in the treatment of 1,000 µM of sodium glycocholate (P<0.05) (Fig. 2D-F).
To evaluate the impact of bile acids to osteoclastogenesis induced by Pg-LPS, RANKL, and M-CSF in RAW 264.7 cells, the cells treated with the stimulators and bile salts for 4 days were stained by TRAP method. TRAP-positive multinucleated cells (with ≥3 nuclei) were regarded as osteoclast or OLCs. In this experiment, RAW 264.7 cells stimulated by Pg-LPS were not differentiated to the TRAP-positive cells. On the other hand, in the groups activated by RANKL with M-CSF, the cells were differentiated to multinucleated giant cells and stained TRAP-positively. However, the amounts of osteoclast or OLCs in the groups added with bile acids were no significant difference compared to those of only RANKL and M-CSF group, except a part of the treatment of sodium glycocholate (Fig. 3A-C). The sodium glycocholate at the highest concentration of 1,000 µM remarkably suppressed osteoclast differentiation of RAW 264.7 cells (P<0.001) (Fig. 3C, D), while sodium taurodeoxycholate and sodium taurocholate were no significant effect on the inhibition of osteoclastogenesis (Fig. 3A, B).
This study aimed to examine the inhibitory effect of bile acids on the inflammatory response and osteoclastogenesis in myelomonocytic cells RAW 264.7 stimulated by periodontopathogen P. gingivalis-LPS and RANKL. The study assessed the effect of bile acids on the cells related to both immune and bone metabolisms as considering periodontal inflammation. RAW 264.7 cells were used because they could differentiate macrophage related inflammation or osteolytic cells. Although several studies evaluated the effect of bile acids on other immune cells and inflammatory disease models such as atopic dermatitis, ulcerative colitis, and sepsis, there was no study to examine the effect on periodontitis or osteoclast differentiation. This study first evaluated osteoclast differentiation because the process of bone loss accompanied by an inflammatory response is critical at periodontitis.
In the test for the cytotoxic effect of bile salts on RAW 264.7 cells, there is no toxic effect to the cells at the most concentration; sodium taurodeoxycholate and sodium taurocholate presented non-cytotoxic at the concentration of equal to or less than 200 µM, while sodium glycocholate did at the maximal concentration of 4,000 µM. Thus, the sodium glycocholate can be applied in a broader range of concentration. The results correspond to the previous study reporting that glycocholate had the anti-inflammatory effect without cytotoxicity within a range of 0.01-1 mg/ml (about 20-2,000 µM) on other immune cells and the most appropriate concentration of taurodeoxycholate applied to bone marrow-derived immune cells was 0.05 mg/ml (about 100 µM)18). On the other hand, taurodeoxycholate at 500-1,000 µM had no toxic effect to intestinal epithelial cell lines and rather promoted their proliferation in another study23). It may be due to differences in the types of cells (immune cells or epithelial cells) and their cell signal mechanisms.
To assess the inhibition of three bile acids to inflammatory responses, the levels of the released pro-inflammatory cytokines IL-6 as well as TNF-α were measured in the conditions combined with Pg-LPS and three bile salts. The expression of IL-6 decreased as the concentration of bile salts increases, and particularly did significantly in sodium taurodeoxycholate and sodium glycocholate. Thus, this study confirmed these bile acids had the inhibitory effect on IL-6 in the immune cells stimulated by a periodontopathogen Pg-LPS. Several studies have reported these bile acids had the anti-inflammatory effect of reducing TNF-α and IL-1β in other tissues including inflamed colon tissue, cutaneous tissue as well as immune cells under another type of LPS stimulation18,19,21). The study also presented a similar anti-inflammatory effect on myelomonocytic immune cells activated by Pg-LPS. However, the effect was stronger to IL-6 than TNF-α. The inhibition levels of TNF-α correspond the study with primary macrophages, where the treatment of glycocholate at 1,000 µM decreased the expression levels of TNF-α to about 50% compared to the control18). Meanwhile, taurodeoxycholate at 100 µM, which was suggested as the optimal concentration in another study, had a weak effect to TNF-α in this study18). Considering the concentration with non-cytotoxic effect, taurodeoxycholate and especially glycocholate are effective to the inhibition of inflammatory mediators.
This study also first examined whether the bile acids suppress osteoclast differentiation from the precursor RAW 264.7 cells. Pg-LPS did not induce RAW 264.7 cells to OLCs, unlike the other study to do with E. coli LPS25). Meanwhile, the cells were differentiated to OLCs under RANKL stimulation. However, the inhibitory effect of bile salts to osteoclastogenesis is weaker than to inflammation. The only sodium glycocholate at 1,000 µM remarkably inhibited osteoclast differentiation, and sodium taurodeoxycholate and sodium taurocholate did not.
In the other study, sodium glycocholate inhibited the expression of a transcription factor NF-κB related to IL-2 as well as the production of TNF-α in the immune cells stimulated by E. coli LPS18). Since NF-κB is expressed in the canonical pathway by TNF-α or RANKL, contributing to induce the osteoclast precursors to osteoclast, the osteoclast differentiation is also suppressed as an NF-κB expression is inhibited. Therefore, the inhibition of osteoclast differentiation by sodium glycocholate presented in this study, might be attributed to the suppression of RANKL-derived NF-κB pathway by the bile acids. Meanwhile, the osteoclast differentiation was inhibited at a relatively high concentration in the study, which also corresponds the result that the activation of NF-κB decreased to 25-75% of the control group at 1,000-2,000 µM of sodium glycocholate18). Thus, the required concentration of glycocholate for anti-osteoclastogenesis is thought to be higher than that for anti-inflammatory effect. On the other hand, taurodeoxycholate activated NF-κB and inhibited subsequent TNF-α-induced apoptosis in intestinal epithelial cells23) and induced the increase of IL-2 in another study19). Therefore, it could be estimated that taurodeoxycholate did not affect to inhibit osteoclast differentiation because it did not interrupt the activation of RANKL-induced NF-κB as opposed to glycocholate. Also, it is possible that taurocholate has a similar mechanism to taurodeoxycholate.
As comprehensively considering cytotoxicity, anti-inflammatory effect, and inhibitory effect on osteoclastogenesis, glycocholate of three bile acids is regarded to be the most effective. However, the concentration of it needed to present the significant effect was relatively high as about 10-1,000 µM, although it had no cytotoxic effect at that concentration, compared to that the effective concentrations of test compounds were about 1-100 µMs in other studies for RAW 264.7 differentiation25,26). Thus, the effectiveness of glycocholate to TNF-α and osteoclast differentiation except IL-6 were not higher than other substances. However, the further studies are needed for glycocholate to reassure and determine the effective and proper concentration, and to elucidate the mechanisms in details for the inhibitory effect on inflammation and osteoclastogenesis related to an NF-κB pathway or other cell signaling. Although other two bile acids with a taurine group had a partial impact on the regulation of inflammation, the studies for them are also needed. However, comprehensively, these bile acids presented inhibitory effect on inflammation and related metabolism induced periodontal pathogens as well as other tissues or systems. The inflammation is common mechanism linking periodontitis and many systemic diseases. Application of bile acids could target inflammation locally periodontally and systemically. Bile acids derivates or extracts used for other purposes could be used in periodontal tissue.
There are several limitations of the study. First, it is needed to set up the environment more similar to periodontal tissue and periodontitis. To test the effect of substances, several studies used periodontal related cell sources such as human gingival fibroblast2) or mouse bone marrow macrophages (BMMs)4,27). However, this study did not use periodontium-origin cell or primary cells. Instead, periodontal pathogen Pg-LPS2,26,27) and myelomonocytic cell lines developing both inflammatory cells (macrophages) and osteoclast25), were used similarly to some studies. RAW 264.7 cells were also confirmed to be developed to osteoclast, although the differentiation of BMMs is definitive. The application to other periodontium-related cells or tissues is needed. Second, there were insufficient results related to anti-osteoclastogenesis and mechanistic explanations involved in inflammatory response and osteoclast differentiation. Therefore, further studies are needed to evaluate the effect of glycocholate mainly and the other bile salts on the inflammatory osteolytic activities in periodontal environments in detail. Particularly, the interest and further studies whether they have different mechanisms with RANKL, NF-κB, and other osteoclastogenesis-related signaling pathway are important since these bile acids presented the contrary effects on the differentiation to osteoclast. In addition, based on the results in vitro study, the further studies including in vivo or other conditions are needed.
This study was the first step to evaluate the potential of bile acids for the prevention or intervention of periodontal inflammation and bone resorption as well as its potential as a universal immuno-regulator. This study was to present that bile acids had inhibitory effects to the inflammation, which is common mechanism between systemic diseases and periodontal diseases. In conclusion, three bile acids inhibited P. gingivalis LPS-induced inflammatory responses and glycocholate partially suppressed RANKL-mediated osteoclastogenesis in RAW 264.7 cells.
Notes
References
1. Yamamoto T, Kita M, Oseko F, Nakamura T, Imanishi J, Kanamura N. 2006; Cytokine production in human periodontal ligament cells stimulated with Porphyromonas gingivalis. J Periodontal Res. 41:554–559. DOI: 10.1111/j.1600-0765.2006.00905.x. PMID: 17076781.

2. Kong L, Qi X, Huang S, Chen S, Wu Y, Zhao L. 2015; Theaflavins inhibit pathogenic properties of P. gingivalis and MMPs production in P. gingivalis-stimulated human gingival fibroblasts. Arch Oral Biol. 60:12–22. DOI: 10.1016/j.archoralbio.2014.08.019. PMID: 25244614.

3. Lee JH, Kim H, Shim JH, Park J, Lee SK, Park KK, et al. 2018; Platycarya strobilacea leaf extract inhibits tumor necrosis factor-a production and bone loss induced by Porphyromonas gingivalis-derived lipopolysaccharide. Arch Oral Biol. 96:46–51. DOI: 10.1016/j.archoralbio.2018.08.011. PMID: 30172945.
4. Lee CT, Huang YW, Zhu L, Weltman R. 2017; Prevalences of peri-implantitis and peri-implant mucositis: systematic review and meta-analysis. J Dent. 62:1–12. DOI: 10.1016/j.jdent.2017.04.011. PMID: 28478213.

5. Sanz-Martin I, Doolittle-Hall J, Teles RP, Patel M, Belibasakis GN, Hämmerle CHF, et al. 2017; Exploring the microbiome of healthy and diseased peri-implant sites using Illumina sequencing. J Clin Periodontol. 44:1274–1284. DOI: 10.1111/jcpe.12788. PMID: 28766745. PMCID: PMC7209775.

6. Ebersole JL. 2003; Humoral immune responses in gingival crevice fluid: local and systemic implications. Periodontol 2000. 31:135–166. DOI: 10.1034/j.1600-0757.2003.03109.x. PMID: 12657000.

7. Seymour GJ, Ford PJ, Cullinan MP, Leishman S, Yamazaki K. 2007; Relationship between periodontal infections and systemic disease. Clin Microbiol Infect. 13 Suppl 4:S3–10. DOI: 10.1111/j.1469-0691.2007.01798.x. PMID: 17716290.

8. Andrukhov O, Ulm C, Reischl H, Nguyen PQ, Matejka M, Rausch-Fan X. 2011; Serum cytokine levels in periodontitis patients in relation to the bacterial load. J Periodontol. 82:885–892. DOI: 10.1902/jop.2010.100425. PMID: 21138356.

9. Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. 2014; Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 105:141–150. DOI: 10.1016/j.diabres.2014.04.006. PMID: 24798950.

10. Nibali L, Tatarakis N, Needleman I, Tu YK, D'Aiuto F, Rizzo M, et al. 2013; Clinical review: Association between metabolic syndrome and periodontitis: a systematic review and meta-analysis. J Clin Endocrinol Metab. 98:913–920. DOI: 10.1210/jc.2012-3552. PMID: 23386648.
11. Sabharwal A, Gomes-Filho IS, Stellrecht E, Scannapieco FA. 2018; Role of periodontal therapy in management of common complex systemic diseases and conditions: An update. Periodontol 2000. 78:212–226. DOI: 10.1111/prd.12226. PMID: 30198128.

12. Papi P, Letizia C, Pilloni A, Petramala L, Saracino V, Rosella D, Pompa G. 2018; Peri-implant diseases and metabolic syndrome components: a systematic review. Eur Rev Med Pharmacol Sci. 22:866–875. DOI: 10.26355/eurrev_201802_14364. PMID: 29509232.
13. Ma MM, Li Y, Liu XY, Zhu WW, Ren X, Kong GQ, et al. 2015; Cyanidin-3-O-Glucoside Ameliorates Lipopolysaccharide-Induced Injury Both In Vivo and In Vitro Suppression of NF-κB and MAPK Pathways. Inflammation. 38:1669–1682. DOI: 10.1007/s10753-015-0144-y. PMID: 25752620.

14. Doyle CJ, Fitzsimmons TR, Marchant C, Dharmapatni AA, Hirsch R, Bartold PM. 2015; Azithromycin suppresses P. gingivalis LPS-induced pro-inflammatory cytokine and chemokine production by human gingival fibroblasts in vitro. Clin Oral Investig. 19:221–227. DOI: 10.1007/s00784-014-1249-7. PMID: 24806810.

15. Panahipour L, Nasserzare S, Amer Z, Brücke F, Stähli A, Kreissl A, et al. 2019; The anti-inflammatory effect of milk and dairy products on periodontal cells: an in vitro approach. Clin Oral Investig. 23:1959–1966. DOI: 10.1007/s00784-018-2642-4. PMID: 30238412.

16. Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall HU, Bamberg K, et al. 2013; Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 17:225–235. DOI: 10.1016/j.cmet.2013.01.003. PMID: 23395169.

17. Copple BL, Li T. 2016; Pharmacology of bile acid receptors: Evolution of bile acids from simple detergents to complex signaling molecules. Pharmacol Res. 104:9–21. DOI: 10.1016/j.phrs.2015.12.007. PMID: 26706784. PMCID: PMC4900180.

18. Seong SY, Kang CG. 2007. Korean intellectual property office;Daejeon: Korea Patent Application No. 10-2007-0046579.
19. Seong SY, Kim YH. 2014. Korean intellectual property office;Daejeon: Korea Patent Application No. 10-2014-0125691.
20. Seong SY, Jang SH, Kim YH, Kim YJ, Jung HE. 2013. Korean intellectual property office;Daejeon: Korea Patent Application No. 10-2013-0101064.
21. Yang Y, He J, Suo Y, Lv L, Wang J, Huo C, et al. 2016; Anti-inflammatory effect of taurocholate on TNBS-induced ulcerative colitis in mice. Biomed Pharmacother. 81:424–430. DOI: 10.1016/j.biopha.2016.04.037. PMID: 27261622.

22. Choi HJ, Yun JW, Kim YH, Kwon E, Hyon MK, Kim JY, et al. 2019; Evaluation of acute and subacute toxicity of sodium taurodeoxycholate in rats. Drug Chem Toxicol. 1–9. DOI: 10.1080/01480545.2019.1609493. PMID: 31215257.

23. Toledo A, Yamaguchi J, Wang JY, Bass BL, Turner DJ, Strauch ED. 2004; Taurodeoxycholate stimulates intestinal cell proliferation and protects against apoptotic cell death through activation of NF-kappaB. Dig Dis Sci. 49:1664–1671. DOI: 10.1023/B:DDAS.0000043383.96077.99. PMID: 15573924.
24. Guo C, Qi H, Yu Y, Zhang Q, Su J, Yu D, et al. 2015; The G-Protein-Coupled Bile Acid Receptor Gpbar1 (TGR5) Inhibits Gastric Inflammation Through Antagonizing NF-κB Signaling Pathway. Front Pharmacol. 6:287. DOI: 10.3389/fphar.2015.00287. PMID: 26696888. PMCID: PMC4675858.

25. Bian T, Li L, Lyu J, Cui D, Lei L, Yan F. 2016; Human b-defensin 3 suppresses Porphyromonas gingivalis lipopolysaccharide-induced inflammation in RAW 264.7 cells and aortas of ApoE-deficient mice. Peptides. 82:92–100. DOI: 10.1016/j.peptides.2016.06.002. PMID: 27298203.
26. Kats A, Norgård M, Wondimu Z, Koro C, Concha Quezada H, Andersson G, et al. 2016; Aminothiazoles inhibit RANKL- and LPS-mediated osteoclastogenesis and PGE2 production in RAW 264.7 cells. J Cell Mol Med. 20:1128–1138. DOI: 10.1111/jcmm.12814. PMID: 26987561. PMCID: PMC4882984.
27. Lee JY. 2017. Effects of bismuth oxide, a component of mineral trioxide aggregate, on osteoclast differentiation [master's thesis]. Seoul national university;Seoul:
Fig. 1
Effect of bile acids on the viability of RAW 264.7 cells. RAW 264.7 cells (3×103 cells/well) were seeded in 96 well-plates and treated with culture medium alone (control) or in combination with bile salts. After 24 hours, the cell viabilities represented as optical density at 570 nm were measured by MTT-assay. (A, B) Sodium taurodeoxycholate and sodium taurocholate were treated at the range of 0-1,000 µM. The cell viabilities of them were no significant differences with that of the control group. However, both groups presented cytotoxicity to the cells 500 and 1,000 µM (P<0.001). (C) Sodium glycocholate treated at the range of 0-4,000 µM presented no cytotoxic effect up to a maximal 4,000 µM. Results were expressed as the relative cell viabilities (%) compared to the control group (no bile salt; 0 µM) as a reference. Bar and error bar present the mean±S.D. of % cell viability relative to the control. ***P<0.001 significantly different from the control. Each point is repeatedly measured.
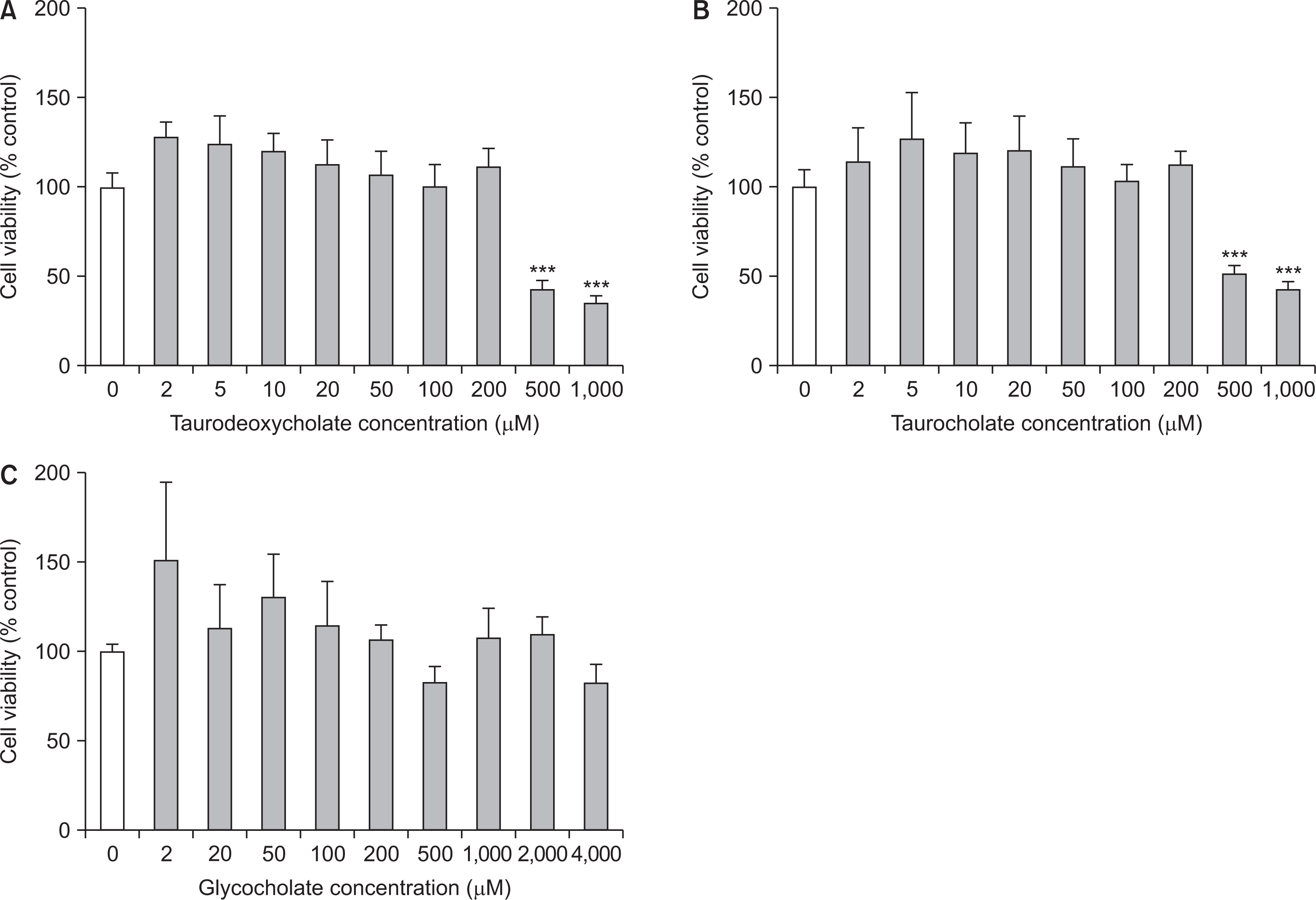
Fig. 2
Effects of three bile acids on expression of pro-inflammatory cytokines in Pg-LPS-stimulated RAW 264.7 cells. RAW 264.7 cells were cultured at a density of 3×104 cells/well in 6-well plates and treated with either culture medium alone (control) and three bile salts with the serial concentrations in the range of 0-100 µM or 0-1,000 µM under the stimulation of Pg-LPS (1 µg/ml) for 12 hours. The levels of pro-inflammatory cytokines IL-6 and TNF-α in the culture supernatants were measured by ELISA. The production of IL-6 (A-C) and TNF-α (D-F) increased significantly under Pg-LPS stimulation alone compared to the control, while the expression of them tended to be decreased with increasing treated concentration of three bile salts in a dose-dependent manner compared to only Pg-LPS group. (A-C) The production of IL-6 was strongly inhibited at concentrations equal to or higher than 100 µM of sodium taurodeoxycholate or 10 µM of sodium glycocholate treatment, each (P<0.001). (D-F) The production of TNF-α was suppressed as the concentration of three bile salts increased although the significance was relatively weak than IL-6. The levels of TNF-α were significantly lowered only in the treatment of sodium glycocholate at 1,000 µM (P<0.05). Results (bar and error bar) present the mean±S.D. of cytokine levels (pg/ml). *P<0.05 , **P<0.01, and ***P<0.001 mean significant differences from Pg-LPS only group. ‘a’ means no significant differences with the control (no Pg-LPS) group. Each point is repeatedly measured (n=6 well in treatment, n=3 in ELISA). Pg-LPS, Porphyromonas gingivalis lipopolysaccharide; IL-6, interleukin-6; TNF-α, tumor necrosis factor-a; ELISA, enzyme-linked immunosorbent assay.
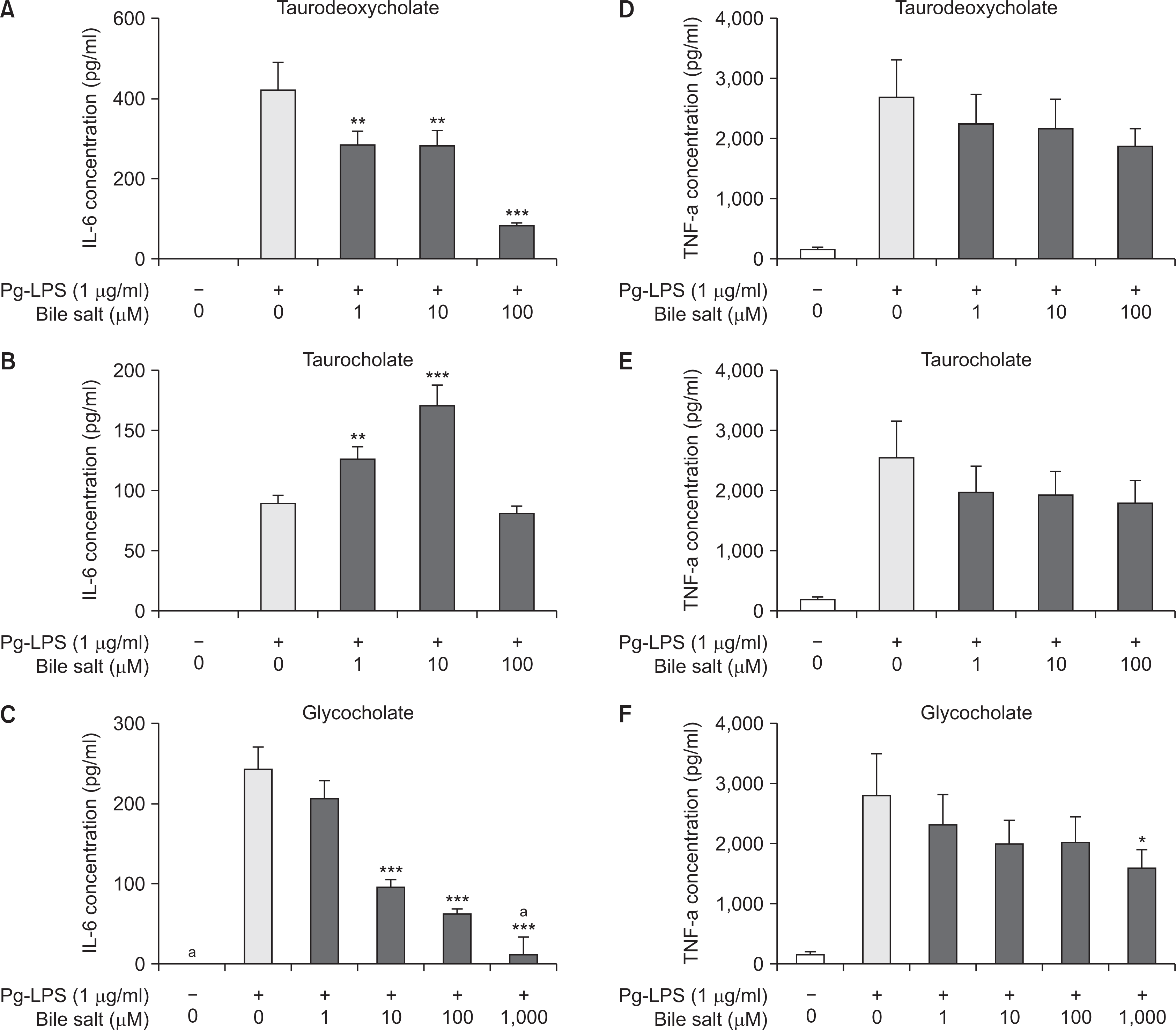
Fig. 3
Effects of three bile acids on osteoclast differentiation of RAW 264.7 cells stimulated by RANKL. (A-C) RAW 264.7 cells (2×103 cells/well) seeded in 96-well plates and treated with RANKL (100 ng/ml) combined with M-CSF (30 ng/ml) in the absence or presence of three bile salts in the range of 0-100 or 0-1,000 µM. After 4 days, the fixing of cells and TRAP staining was carried out. TRAP-positive multinucleated cells (with ≥3 nuclei) were regarded as osteoclast or OLCs. The treatment of RANKL and M-CSF (only RANKL group) stimulated the cells to differentiate to multinucleated cells as osteoclast or OLCs. However, the bile salts treatments did not suppress osteoclast differentiation of the cells at most concentrations. (A, B) While sodium taurodeoxycholate and sodium taurocholate were no effect on the inhibition of osteoclastogenesis, (C) sodium glycocholate at 1,000 µM (the highest concentration) significantly suppressed osteoclast differentiation of RAW 264.7 cells (P<0.001) (D) as showed in the pictures of TRAP staining for sodium glycocholate treatment group. The RANKL only group presented many multinucleated giant cells as the cells indicated red arrows, while the cells with 1,000 µM sodium glycocholate were similar to ones of the control. Results were expressed by relative number (ratio) of TRAP positive-OLCs to the only RANKL group. Bar and error bar present the mean±S.D. of relative ratio to the only RANKL without bile salt as a reference value (1.00). ***P<0.001 significantly different from the only RANKL group. Each point is repeatedly measured (n=4). RANKL, activating receptor activator of nuclear factor-κB ligand; M-CSF, macrophage colony-stimulating factor; TRAP, tartrate-resistant acid phosphatase; OLCs, osteoclast-like cells.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download