I. Introduction
Since its initial description
1, the incidence of medication-related osteonecrosis of the jaw (MRONJ) and the number of related publications have increased rapidly. As this serious disease
2 has been studied by clinicians and scientists, controversies have arisen between medical societies
3-6.
The Advisory Task Force on Bisphosphonate-Related Osteonecrosis of the Jaw from the American Association of Oral and Maxillofacial Surgeons (AAOMS) has made great efforts to present an accepted and widely used definition of MRONJ
7-9. The following definition is the current gold standard for MRONJ worldwide: the presence of exposed jawbone or bone that can be probed for at least 8 weeks in a patient receiving antiresorptive therapy who has not received radiotherapy to the head or neck. Furthermore, AAOMS developed a four-stage disease classification scale (stage 0 to III) that is regarded as the standard for deriving treatment recommendations and creating comparability in the nomenclature of epidemiological data
9.
However, the AAOMS staging system has also been the subject of controversy. The absolute goal of a staging system should be to depict the extent of the disease, derive suitable therapeutic options, and make prognostic assessments. However, the AAOMS staging system, which is driven by clinical inspectorial examinations, might fail to detect the actual extent of bone involvement in MRONJ, raising the risk of assigning patients to inappropriate treatments
10,11. In particular, AAOMS stage I
9 is misleading. Because an early stage implies a good prognosis, the AAOMS recommends that stage I patients be treated with a non-surgical, conservative approach. However, the size of the mucosal defect (sinus tract or bones exposed over a large area) does not allow conclusions to be drawn about the true extent of the necrosis or the appropriate therapy recommendation or prognosis. Indeed, few data are available to support MRONJ staging or the concerns about it. Therefore, it is not surprising that many working groups have attempted to investigate the relationship between the staging and the true extent of necrosis by using radiological diagnostics to detect early signs of MRONJ or assess the real extent of the disease
12-16. Unfortunately, there is no consensus on the efficacy of different imaging modalities in assessing the real extent of necrotic bone area
17,18. Furthermore, inconsistent results have been found, including both over- and underestimation of the extent of MRONJ when comparing the AAOMS stages with various imaging modalities
19. At present, it seems that determining the true extent of necrosis is only possible intraoperatively using the judgment of experienced surgeons or fluorescence-based methods
20-22.
To date, no clinical data investigating the connection between the size of a mucosal defect and the extent of necrosis have been published. Before it makes sense to question a clinically established classification to improve it, it is urgently necessary to provide evidence that the classification is clinically relevant. Therefore, our aims in this study were to determine whether the extent of a mucosal lesion correlates with the bony lesion in stage I patients. We also wanted to assess possible intragroup differences in the clinical manifestation of necrosis (probeable vs visible bone). We hypothesize that the extent of the mucosal lesion bears no relationship to the bony lesion and that whether the necrotic bone is visible or probeable is clinically irrelevant.
Go to :

III. Results
1. Patient data
In total, 55 Caucasian patients with 86 MRONJ lesions were enrolled in this study (36 females [mean age, 68.2±8.5 years] and 19 males [mean age, 72.5±10.4 years]).
Nine patients who were included suffered from osteoporosis, and 46 had an underlying malignancy with metastasis to or primary focus (such as multiple myeloma) on the bone (prostate, 12; breast, 20; multiple myeloma, 5; kidney cell carcinoma, 4; and others, 5).
Of the 55 patients included, 19 were treated with zoledronate, 2 with ibandronat, and 4 with alendronate. Twenty-five patients were treated with denosumab. The remaining 5 patients reported subsequent or alternating intake of bisphosphonates and denosumab. The mean duration of intake for the antiresorptive drugs prior to treatment was 49.3±41.5 months (range, 8-224 months).
Thirty-one patients showed only one MRONJ lesion, 23 patients had two, and 3 patients had three. Thirty-five lesions were located in the upper jaw, and 51 lesions were located in the lower jaw.
Of the 86 stage I MRONJ
9 lesions, 46 patients had bone visibly exposed to the oral cavity (visible bone group), and 40 lesions could be probed to bone through the sinus tract (probeable bone group).
2. Extent of mucosal lesions, intraoperative necrotic lesions
Detailed results are given in
Tables 1 and
2 and
Fig. 3. In the visible bone group, the mucosal lesion in both the anteroposterior and transversal directions decreased from the day of inclusion (T0) (anteroposterior dimension, 11.2±9.0 mm; transversal, 7.7±6.5 mm) to the day of surgery (anteroposterior dimension, 10.2±8.3 mm; transversal, 7.1±5.7 mm). However, those differences are not significant.
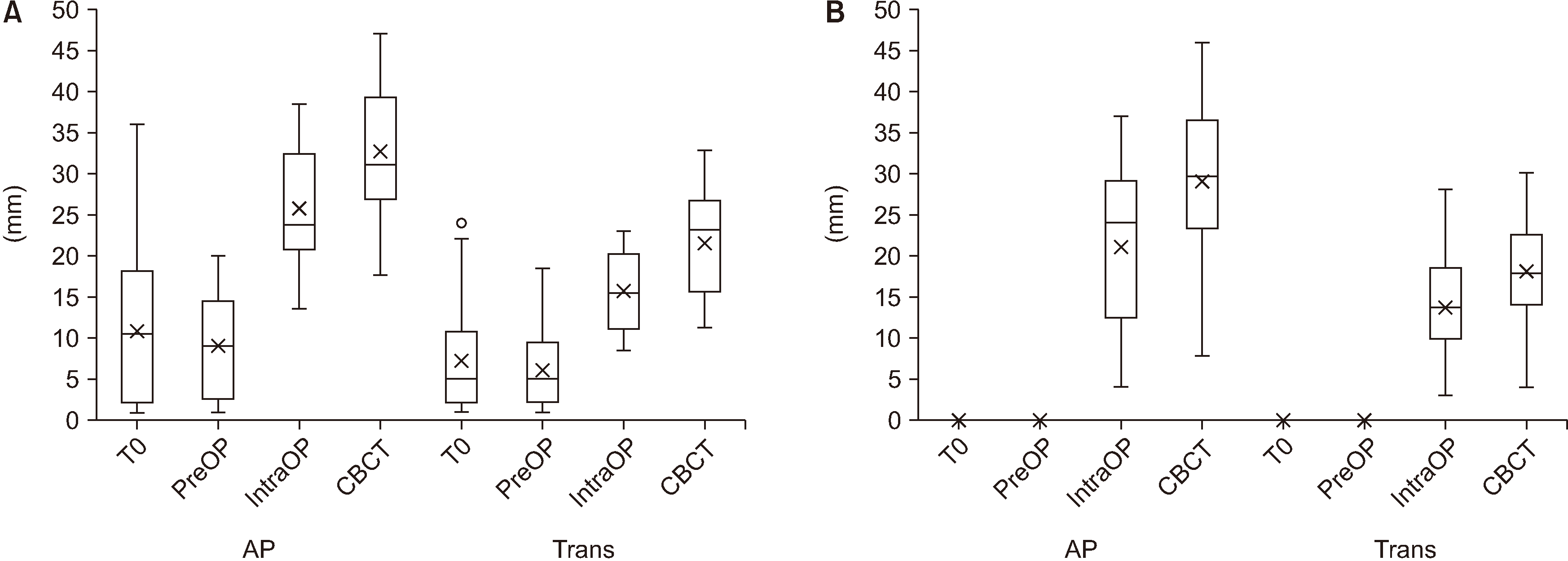 | Fig. 3Boxplot comparing the anteroposterior (AP) and transversal (Trans) extent of: the mucosal lesion at presentation (T0), the mucosal lesion on the day of surgery (PreOP), the extent of the necrotic bone intraoperatively (IntraOP), and the extent of the resection defect measured by cone-beam computed tomography (CBCT) in the visible bone group (A) and probeable bone group (B). 
|
Table 1
Descriptive statistics indicating the extent of the lesions
|
Group |
Direction |
Measurement |
No. of patients |
Value (mm) |
|
Visible bone group |
Anteroposterior |
Mucosal lesion 4 weeks prior to surgery |
46 |
11.2±9.0 (1.0-36.0) |
|
|
Mucosal lesion on day of surgery |
46 |
10.2±8.3 (1.0-30.0) |
|
|
Osseous necrotic lesion intraoperatively |
46 |
27.4±12.2 (10.0-75.0) |
|
|
Resection defect (measured radiologically) |
31 |
33.6±8.6 (17.6-53.5) |
|
Transversal |
Mucosal lesion 4 weeks prior to surgery |
46 |
7.7±6.5 (1.0-24.0) |
|
|
Mucosal lesion on day of surgery |
46 |
7.1±5.7 (1.0-20.0) |
|
|
Osseous necrotic lesion intraoperatively |
46 |
17.1±7.2 (8.5-50.0) |
|
|
Resection defect (measured radiologically) |
31 |
21.8±6.1 (11.3-32.8) |
|
Probeable bone group |
Anteroposterior |
Mucosal lesion 4 weeks prior to surgery |
40 |
0 |
|
|
Mucosal lesion on day of surgery |
40 |
0 |
|
|
Osseous necrotic lesion intraoperatively |
40 |
20.8±9.4 (4.0-37.0) |
|
|
Resection defect (measured radiologically) |
32 |
29.0±10.5 (7.8-45.8) |
|
Transversal |
Mucosal lesion 4 weeks prior to surgery |
40 |
0 |
|
|
Mucosal lesion on day of surgery |
40 |
0 |
|
|
Osseous necrotic lesion intraoperatively |
40 |
15.4±9.0 (3.0-55.0) |
|
|
Resection defect (measured radiologically) |
32 |
18.0±6.5 (4.0-30.1) |

Table 2
Two-tailed t-test between different time points
|
Group |
Direction |
Measurement (1) |
Measurement (2) |
Value (mm) |
t
|
df |
P (2-tailed) |
|
Visible bone group |
Anteroposterior |
Mucosal lesion 4 weeks prior to surgery |
Mucosal lesion on day of surgery |
0.98±4.23 |
1.57 |
45 |
0.124 |
|
|
Mucosal lesion on day of surgery |
Osseous necrotic lesion intraoperatively |
–17.24±11.70 |
–9.99 |
45 |
0.000*
|
|
|
Osseous necrotic lesion intraoperatively |
Resection defect (measured radiologically) |
–7.52±4.09 |
–10.23 |
30 |
0.000*
|
|
Transversal |
Mucosal lesion 4 weeks prior to surgery |
Mucosal lesion on day of surgery |
0.65±3.40 |
1.30 |
45 |
0.200 |
|
|
Mucosal lesion on day of surgery |
Osseous necrotic lesion intraoperatively |
–9.98±7.28 |
–9.30 |
45 |
0.000*
|
|
|
Osseous necrotic lesion intraoperatively |
Resection defect (measured radiologically) |
–6.05±3.45 |
–9.75 |
30 |
0.000*
|
|
Probeable bone group |
Anteroposterior |
Osseous necrotic lesion intraoperatively |
Resection defect (measured radiologically) |
–7.86±5.02 |
–8.86 |
31 |
0.000*
|
|
Transversal |
Osseous necrotic lesion intraoperatively |
Resection defect (measured radiologically) |
–4.26±2.03 |
–11.90 |
31 |
0.000*
|

In the probeable bone group, mucosal healing did not occur in any patient from T0 until surgery.
When we compared the preoperative mucosal lesion with the extent of the necrotic osseous lesion in the visible bone group, the extent of the osseous lesion (anteroposterior dimension, 27.4±12.2 mm; transversal, 17.1±7.2 mm) was significantly (P<0.001) larger than the mucosal lesion (anteroposterior dimension, 10.2±8.3 mm; transversal, 7.1±5.7 mm).
In the probeable bone group, extensive necrotic lesions also appeared intraoperatively (anteroposterior dimension, 20.8±9.4 mm; transversal, 15.4±9.0 mm).
3. Resection defects
Detailed results are presented in
Tables 1 and
2 and
Fig. 3. In the visible bone group, the resection defect (anteroposterior dimension, 33.6±8.6 mm; transversal, 21.8±6.1 mm) was significantly larger (anteroposterior dimension, T(30)=–10.23,
P<0.001; transversal, T(30)=–9.75,
P<0.001) than the necrotic area (anteroposterior dimension, 27.4±12.2 mm; transversal, 17.1±7.2 mm).
In the probeable bone group, the resection defect (anteroposterior dimension, 29.0±10.5 mm; transversal, 18.0±6.5 mm) was also significantly larger (anteroposterior dimension, T(31)=–8.86, P<0.001; transversal, T(31)=–11.90, P<0.001) than the necrotic osseous area (anteroposterior dimension, 20.8±9.4 mm; transversal, 15.4±9.0 mm).
The CBCT analysis showed that in 6 lesions in 3 patients, the resection defects exceeded the region of alveolar bone and could thus postoperatively be considered stage III lesions.
4. Comparison between groups
Detailed results are given in
Table 3. When we compared the two groups using a two-sample
t-test, the extent of the osseous necrotic lesion in the anteroposterior direction turned out to be significantly (T(84)=2.78;
P=0.007) larger in the visible bone group. No significant difference between the two groups was found for the extent of the osseous lesion in the transversal direction (T(84)=0.96;
P=0.342).
Table 3
Two-tailed t-test between both groups (unit: mm)
|
Timepoint |
Direction |
Visible
bone group |
Probeable
bone group |
∆ |
t-test |
|
|
t
|
df |
P (2-tailed) |
|
Mucosal lesion 4 weeks prior to surgery |
Anteroposterior |
11.2±9.0 |
0 |
11.2±1.44 |
8.10 |
84 |
0.000*
|
|
Mucosal lesion on day of surgery |
|
10.2±8.3 |
0 |
10.2±1.31 |
7.75 |
84 |
0.000*
|
|
Osseous necrotic lesion intraoperatively |
|
27.4±12.2 |
20.8±9.4 |
6.6±2.37 |
2.78 |
84 |
0.007*
|
|
Resection defect (measured radiologically) |
|
33.6±8.6 |
29.0±10.5 |
4.7±2.42 |
1.93 |
61 |
0.058 |
|
Mucosal lesion 4 weeks prior to surgery |
Transversal |
7.7±6.5 |
0 |
7.7±1.04 |
7.67 |
84 |
0.000*
|
|
Mucosal lesion on day of surgery |
|
7.1±5.7 |
0 |
7.1±0.91 |
7.83 |
84 |
0.000*
|
|
Osseous necrotic lesion intraoperatively |
|
17.1±7.2 |
15.4±9.0 |
1.7±1.74 |
0.96 |
84 |
0.342 |
|
Resection defect (measured radiologically) |
|
21.8±6.1 |
18.0±6.5 |
3.7±1.59 |
2.35 |
61 |
0.022*
|

5. Relationships
Table 4 depicts the results of the linear regression analysis of the measurements in both groups.
Table 4
|
Group |
Direction |
Predictor |
Dependent variable |
B |
β |
df |
R2
|
t
|
P-value |
|
Visible bone group |
Anteroposterior |
Mucosal lesion on day of surgery |
Osseous necrotic lesion intraoperatively |
0.583 |
0.397 |
45 |
0.158 |
2.872 |
0.006*
|
|
Mucosal lesion on day of surgery |
Resection defect (measured radiologically) |
0.235 |
0.176 |
30 |
0.031 |
0.963 |
0.343 |
|
Osseous necrotic lesion intraoperatively |
Resection defect (measured radiologically) |
1.015 |
0.880 |
30 |
0.774 |
9.968 |
0.000*
|
|
Transversal |
Mucosal lesion on day of surgery |
Osseous necrotic lesion intraoperatively |
0.473 |
0.378 |
45 |
0.143 |
2.706 |
0.010*
|
|
|
Mucosal lesion on day of surgery |
Resection defect (measured radiologically) |
0.273 |
0.203 |
30 |
0.041 |
1.116 |
0.274 |
|
|
Osseous necrotic lesion intraoperatively |
Resection defect (measured radiologically) |
0.992 |
0.826 |
30 |
0.682 |
7.883 |
0.000*
|
|
Probeable bone group |
Anteroposterior |
Osseous necrotic lesion intraoperatively |
Resection defect (measured radiologically) |
0.927 |
0.880 |
31 |
0.775 |
10.152 |
0.000*
|
|
Transversal |
Osseous necrotic lesion intraoperatively |
Resection defect (measured radiologically) |
1.025 |
0.951 |
31 |
0.904 |
16.797 |
0.000*
|

Using the extent of the mucosal lesion on the day of surgery as the predictor and the extent of the osseous necrotic lesion measured intraoperatively as the dependent variable in the anteroposterior direction in the visible bone group, the overall regression model was significant (T(45)=2.872, P=0.006). Thus, the extent of the mucosal lesion predicted the extent of necrotic bone (B=0.583). However, that linear regression model explains only 15.8% of the variance in the data (R2=0.158). The finding for the extent of necrotic bone and the resection defect was the same.
Go to :

IV. Discussion
Even though great progress has been made in diagnosing and treating MRONJ in recent years, diagnosis (especially early cases) and staging remain challenging, partly because the diagnostic procedure is not standardized, and the AAOMS classification does not cover all manifestations of MRONJ
11,19.
Compared with the 2009 version
7,8, the most recent version of the AAOMS consensus paper has widened the definition of MRONJ
9. This amendment was triggered by several studies that found that patients can suffer from MRONJ without visually detectable necrotic bone (non-exposed MRONJ, including intraoral fistula, mandibular fracture, dentally unexplained pain, and swelling) and would therefore not fulfill the initial definition of MRONJ
10,12,13,24,25. Therefore, the modified definition in the latest AAOMS consensus paper includes patients who present with bone probeable through a sinus tract
9. This change is an important step forward because different interpretations of “bone exposure” had led some authors to diagnose probeable bone as MRONJ, whereas others did not. That disagreement might partly explain the different prevalence figures in recent epidemiological studies
19.
However, the current staging system still suffers from several inconsistencies. In its 2009 consensus paper
8, AAOMS added a “stage 0” to its classification system for subjects with the non-exposed variant of MRONJ (no clinical evidence of necrotic bone but nonspecific clinical findings, radiographic changes, and symptoms). This stage is accompanied by nonspecific symptoms (odontalgia, bone pain in the jaw, loosening of teeth, and others). Unfortunately, the core definition of MRONJ remains unchanged, and AAOMS continues to focus on the clinical evidence of long-standing bony exposure
11. Consequently, patients with non-exposed MRONJ without probeable bone do not have MRONJ as it is currently defined. This paradox has been highlighted by many authors calling for urgent change
13,26-28.
In a hierarchical staging system such as the AAOMS classification (stage 0-III), both clinicians and patients should be able to assume that the stages differ in terms of the extent or severity of disease they represent. However, AAOMS has made no statement about whether their stages correlate with differences in the extent of necrotic bone area
9. That failure was precisely the starting point for this study.
In this study, we have shown that the intraoperatively detected areas of necrotic bone were significantly larger than the preoperative visible mucosal lesions in both the visible and probeable bone groups. However, we also showed that the intraoperatively detected necrotic osseous lesions in stage I patients
9 with mucosal defects and visible bone were significantly larger than those in patients with preoperative probeable bone. Furthermore, our linear regression model shows a weak but significant correlation between the extent of the mucosal lesion and the necrotic bone area in the anteroposterior direction in patients who presented with visible bone preoperatively.
However, the very low correlation coefficients (R2<0.2) indicate that our regression models cannot be used to predict the exact extent of a necrotic bony lesion from the extent of a mucosal lesion. The scattering around the regression line is far too large for that. Instead, it must be assumed that other factors influence the extent of both the necrotic and mucosal lesions. Furthermore, the direction of influence is unclear. For future studies, we highly recommend the use of multivariate regression analyses in a larger population.
A further linear regression model between the preoperative extent of the mucosal lesion and the resection defect did not show significant results. In other words, preoperative patient presentation with visible or probeable bone does not predict the extent of the underlying necrotic osseous lesion. However, large areas of exposed bone might indicate that necrosis is extensive, perhaps because a small mucosal lesion protects necrotic bone from prolonged exposure to bacteria, which limits secondary infections and disease progression.
Furthermore, we have shown that the resection defects were significantly larger than the mucosal lesions, although no regression was detected between the extent of the mucosal lesion and the surgically induced defect. That the resection defect is obviously larger than the mucosal and osseous lesions is not surprising; it is a result of the necessary extraction of adjacent teeth and the smoothing of sharp bone edges after the removal of the necrotic bone. However, our results suggest that it is impossible to use mucosal defects and AAOMS stages
9 to predict the effort needed for the surgical management of MRONJ.
Furthermore, for 6 lesions classified as stage I preoperatively, resections beyond the alveolar bone were needed, meaning that those lesions were postoperatively classified as stage III.
To the best of our knowledge, no previous study has investigated the questions addressed herein. A partly comparable study was conducted by Assaf et al.
29, who compared the extent of MRONJ, as detected by Tc-99m-methylene diphosphonate bone scintigraphy, with the intraoperative extent of the disease. They found that the true extent of osseous lesions, as determined by surgery, was significantly underestimated by clinical examination but not by bone scintigraphy
29. Their results underline the importance of imaging in diagnosing and managing MRONJ.
A criticism from surgeons who treat according to the AAOMS treatment recommendations for MRONJ will certainly be that the patients in the present study who presented with MRONJ stage I
9 were surgically treated. There is currently great controversy between international professional societies regarding the AAOMS treatment recommendations
9. For stages I and II, AAOMS recommends non-surgical treatment
9 such as symptomatic treatment with oral antibiotics, oral antibacterial mouth rinse, pain control, and regular clinical follow-up. Surgical debridement or resection of necrotic bone is recommended for stage II MRONJ patients
9. Similar recommendations were also made by Khan et al.
30 on behalf of the International Osteonecrosis of the Jaw Task Force. They based their treatment recommendations on the disease stage and size of the lesion. In their view, conservative therapy should be continued until obvious progression of the disease occurs, pain cannot be controlled by conservative means, or antiresorptive therapy is discontinued by the treating oncologist
30. However, as the results of this study show, neither the severity nor progression of the disease can be measured or interpreted using the current clinically driven staging system
9. Therefore, we assert that therapy recommendations based on those criteria must be critically reviewed and interpreted.
Furthermore, it must not go unmentioned that non-surgical management in cases of infection (AAOMS stage II
9) through the use of antibacterial treatment usually leads to a stage downshift (to AAOMS stage I)
31,32, which is interpreted as a treatment success by many authors
33. Such a fluent transition between those lower stages does not produce any clear information about the real osseous lesion. In addition to freedom from infection, some authors describe a decline in the mucosal lesion and sometimes even the total rehabilitation of mucosal integrity
33-35. In this study, we observed a reduction in the size of the mucosal lesion between the time of study inclusion (T0) and the day of surgery (T1), but that improvement in the mucosal situation should not be misinterpreted as evidence that the disease has resolved. As can be seen from our intraoperative evaluation, the necrotic lesion was significantly larger than the mucosal lesion, with only a tenuous relationship between their sizes. In other words, the successful elimination of infection and restoration of mucosal integrity neither resolves nor revitalizes the necrotic bone.
Data from a recent longitudinal study by our working group even suggests that necrotic bone defects show a tendency to enlarge rather than diminish
32. In that study, 92 patients with stage I MRONJ
9 were initially treated using a standardized conservative (non-surgical) protocol of antimicrobial mouth rinsing and gel application (with chlorhexidine). Only 8 patients (8.7%) showed complete mucosal healing and resolution of symptoms, whereas the remaining 84 (91.3%) had persistent exposed jawbone at the end of the observation period (15.6 months). Among those 84 patients, 67 (79.8%) showed an upshift in AAOMS stage from I to II or III, which inevitably led to operations, with extensive bone loss in 28 cases
32.
Considering that the management of stage III patients is known to be a major challenge and that outcomes are often worse than in earlier stages
36, the AAOMS treatment recommendations are even more questionable. In a large retrospective cohort using data from more than 10 years, Ruggiero and Kohn
36 observed a significant difference in outcomes with respect to the disease stage, with stage I and II disease showing a higher likelihood of better outcomes than stage III. In addition, they showed that a positive result in stage III was 28 times more likely to be achieved by surgical treatment than by non-surgical treatment
36. Those results stand in total contrast to the recommendations published a year before by that same author
9.
A closer look at our data shows that the necrotic lesion is 2 to 3 times larger than the mucosal lesion. The extent of the necrotic lesion in patients with only probeable bone was also comparable to that in patients with visible bone. Surgically induced resection defects of up to 53 mm in this trial argue in favor of early surgical treatment and against postponing surgical therapy until stage III
9 has been reached. Given that MRONJ patients are usually already seriously ill and in need of rapid continuation of their antiresorptive or oncological therapy, which is delayed by conservative, non-surgical therapy
37,38, several working groups and international guidelines argue in favor of early surgical treatment.
To summarize our findings, the clinically driven AAOMS staging system fails to reflect the actual extent of MRONJ. As a logical consequence, it is not possible to derive correct treatment recommendations from it
11. Knowledge about the extent of necrosis is indispensable to surgical treatment planning for MRONJ. Consequently, some authors recommend the use of different imaging modalities to accurately assess the extent of MRONJ preoperatively
12.
Unfortunately, no consensus has been reached on the efficacy of different radiological imaging modalities in assessing the extent of a necrotic osseous lesion
39. The latest meta-analysis to examine the prevalence of radiographic findings on jaws exposed to antiresorptive therapy, including jaws with MRONJ, identified only 29 studies with a total of 1,133 patients. The most frequent radiological findings were mixed lytic-sclerotic areas (73.88%), osteolytic changes (66.18%), osteosclerosis (65.75%), cortical bone erosion (50.83%), and persisting alveolar socket (45.77%)
39. Those authors emphasized that the overall level of evidence in the studies identified was very low. They also pointed out that no imaging modality currently allows clear differentiation between necrotic and vital bone
39. That is why we assessed the extent of MRONJ in this study using an intraoperative measurement technique.
Certainly, auto-fluorescence-guided resection of necrotic bone has some drawbacks. For instance, bleeding in the operating area makes the fluorescence properties difficult to obtain, which might distort the measurements. It is also difficult to use this technique in a sterile operating theater because no currently available devices allow thermic sterilization
22. However, this technique can assess the extent of necrotic bone with great clinical accuracy
21,22.
Nonetheless, further efforts must be made to find radiographic modalities that can clearly differentiate between necrotic and vital bone. Such modalities could enable early diagnosis, significantly affecting the management of the underlying disease and enabling better treatment and the prevention of new potential cases
39,40. By assessing the real extent of the lesion and detecting asymptomatic necrotic areas, high-resolution imaging could be helpful in individualized treatment planning
41. Such imaging is necessary to define a new classification for MRONJ.
The main limitation of this study is the relatively small number of patients involved, which inevitably limits its statistical power to detect significant associations. Furthermore, only patients with stage I MRONJ
9 were included in the trial. Therefore, these results can only be generalized to other patients to a limited degree. In assessing the extent of mucosal or necrotic lesions or resection defects, the measurement methods allowed only two-dimensional measurements. Because MRONJ is truly a three-dimensional problem, future studies should find a way to make three-dimensional measurements. The heterogeneous intake of antiresorptive drugs, as well anti-angiogenetic or immunomodulatory medications, might also be a limitation because our sample size was too small to allow subgroup analyses that could highlight potential differences caused by the type and administration of medications. Indeed, subgroup analyses in multifactorial regression models might be the aim of future prospective clinical trials. This research has brought to light many questions that need further investigation. Future studies should use a prospective approach to analyze a larger group of patients with all stages of MRONJ.
Go to :







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download