I. Introduction
After tooth extraction, the alveolar bone undergoes dimensional and structural alterations. These changes are associated with decreased blood supply from the periodontal ligament, resulting in a marked increase in osteoclastic activity
1. Consequently, the bundle bone, which is a tooth-dependent structure, gradually decreases, and the reduction is more pronounced in the buccal side than in the lingual side
2. In addition, in the edentulous area, which does not have long-term functional power, the bone mass becomes insufficient when absorption of the alveolar bone continues due to nonuse, resulting in atrophy. In this case, the quality of bone becomes poorer and the amount of soft tissue is reduced
3. These changes are often irreversible, thus impeding functional and aesthetic implantation.
Guided bone regeneration (GBR) surgery is one of the best established methods for augmenting the alveolar bone before or during implant placement. Flap dehiscence, membrane exposure, and poor quality and quantity of the regenerated bone are common complications. To prevent these complications, tension-free primary closure is a prerequisite for successful GBR surgery
4. A periosteal releasing incision is a predictable and easy way to advance the flap when the soft tissue is insufficient to achieve complete coverage
5,6. However, as the extent of release increases, the degree of hematoma, swelling, and pain also increase which has a significant effect on the quality of life of patients after surgery and can lead to surgical results that are not ideal
6.
Edema is caused by the accumulation of serous fluid in the interstitial space in response to surgical trauma. The degree of edema depends on the patient, surgical method, degree of invasive surgery, and length of surgical intervention. Edema early during the healing process can cause severe pain to the patient and may lead to dehiscence of the predicate, resulting in delayed healing. Various drugs and methods have been used to reduce postoperative edema, such as cold therapy, low-level laser therapy, and steroid and nonsteroidal anti-inflammatory agents.
Hyaluronic acid (HA) is a major component of the interstitial barrier and has strong hydrophilic and hydration activities. HA prevents the regression of extravascular fluid to the lymphatic system, thus contributing to the maintenance of edematous conditions
7. Hyaluronidase is an enzyme that exerts an anti-inflammatory effect and is present in connective tissue and extracellular matrix
8. Hyaluronidase, which acts as a spreading factor, degrades HA to increase the local intracellular permeability of the peripheral lymphatic channel and reduce viscosity. Reportedly, these properties allow the spreading of fluid inside the interstitial space and help reduce edema
9. In addition, the diffusion capacity of hyaluronidase increases the analgesic efficacy of local anesthetics and helps reduce postoperative pain
10.
In the present study, topical hyaluronidase was applied when performing GBR to improve the hard tissue condition prior to implant placement. Furthermore, we evaluated whether application of hyaluronidase is effective at reducing postoperative edema and pain.
Go to :

II. Materials and Methods
This prospective, randomized, controlled clinical trial targeted patients with severe bone defects in the maxillary and mandibular posterior regions who required a bone graft before placement at Seoul National University Bundang Hospital between January 2018 and October 2018 (clinical trial registration No. 06-2017-198; registration date December 9, 2017). This study was conducted with the approval of the Bioethics Review Committee of Seoul National University Bundang Hospital (B-1708-415-005). The research was performed in adherence with the central tenets of the Declaration of Helsinki. To protect patient confidentiality, special care was taken to avoid exposing the patient’s personal information and face. Informed consent was obtained from all participants and all research was performed in accordance with the relevant guidelines.
At the beginning of the trial, we aimed to evaluate 20 patients in each group. The sample size was calculated based on health medicine statistics. We intended to achieve 80% power and a 5% significance level, assuming a dropout rate of 20%. However, during the trial period, two patients in the trial group were excluded because they were unable to follow the study protocol.
The inclusion criteria were as follows: bone graft required before maxillary and mandibular posterior implant surgery; 20 years of age or older with complete jaw bone growth; written informed consent to participate in this clinical trial; voluntary decision to participate in this clinical trial and written informed consent provided; well-controlled medical conditions (diabetes, hypertension, heart disease) managed with standard treatment; eligible to participate in clinical trials; and judged as suitable for participation in the clinical trial by the responsible researcher.
Exclusion criteria were as follows: autoimmune diseases adversely affecting bone metabolism; systemic diseases not medically controlled; suspected or demonstrated mental illness; pregnancy or lactating; abusive alcohol consumption; and judged by the clinical researcher as not suitable for participation in the clinical trial.
Thirty-eight patients were randomly assigned to the control group (GBR sites untreated; n=20) or the test group. The test group received liquid hyaluronidase (Hirax; BMI Korea, Jeju, Korea) 1 mL; 0.5 mL was directly injected in the mesial area and 0.5 mL was directly injected in the distal area of the GBR sites (n=18) immediately after suturing was completed. The randomization results were kept confidential for all subjects.
Among the 20 controls, 10 patients underwent surgery of the maxilla and the other 10 underwent surgery of the mandible. Eleven of the 18 patients in the test group underwent surgery of the maxilla and the remaining 7 underwent surgery of the mandible. The 38 patients who agreed to participate in the study were between 31 and 79 years of age (mean age, 59.2 years). Among the 20 controls, 15 were male and 5 were female. The mean age of the controls was 61.55 years. Among the 18 participants in the test group, 11 were male and 7 were female, with a mean age of 57.7 years.(
Table 1)
Table 1
Demographic characteristics
|
Variable |
Control group
(n=20) |
Test group
(n=18) |
|
Sex |
|
|
|
Male |
15 (75.0) |
11 (61.1) |
|
Female |
5 (25.0) |
7 (38.9) |
|
Mean age (yr) |
61.55 |
57.05 |
|
Site |
|
|
|
Maxilla |
10 (50.0) |
11 (61.1) |
|
Mandible |
10 (50.0) |
7 (38.9) |

The primary outcome was the degree of edema reduction, and the secondary outcome was the degree of pain reduction. Postoperative swelling was evaluated by measuring the length of the line connected to the specific landmark of the face immediately after surgery (T1) and 2-4 days after surgery (T2). After using an oil pen to draw a landmark on the patient’s face, a moisture-proof band was used to prevent the landmark from being erased. A flexible ruler was used to measure the distance between landmarks (recorded in millimeters). Measurement points included the tragus (T), mouth corner (MC), nasal alar (NA), mandible angle (MA), and outer canthus (OC). Six different measurements were recorded between the landmarks (
Fig. 1): T-MC, T-NA, MA-MC (horizontal distances) and MA-NA, MA-OC, OC-MC (vertical distances). The following equation was used to obtain the percentage of facial swelling: percentage of facial swelling=difference between the T2 and T1 values, divided by the T1 value, and multiplied by 100
(T2 value−T1 valueT1 value×100), as described by Cerqueira et al.
11.
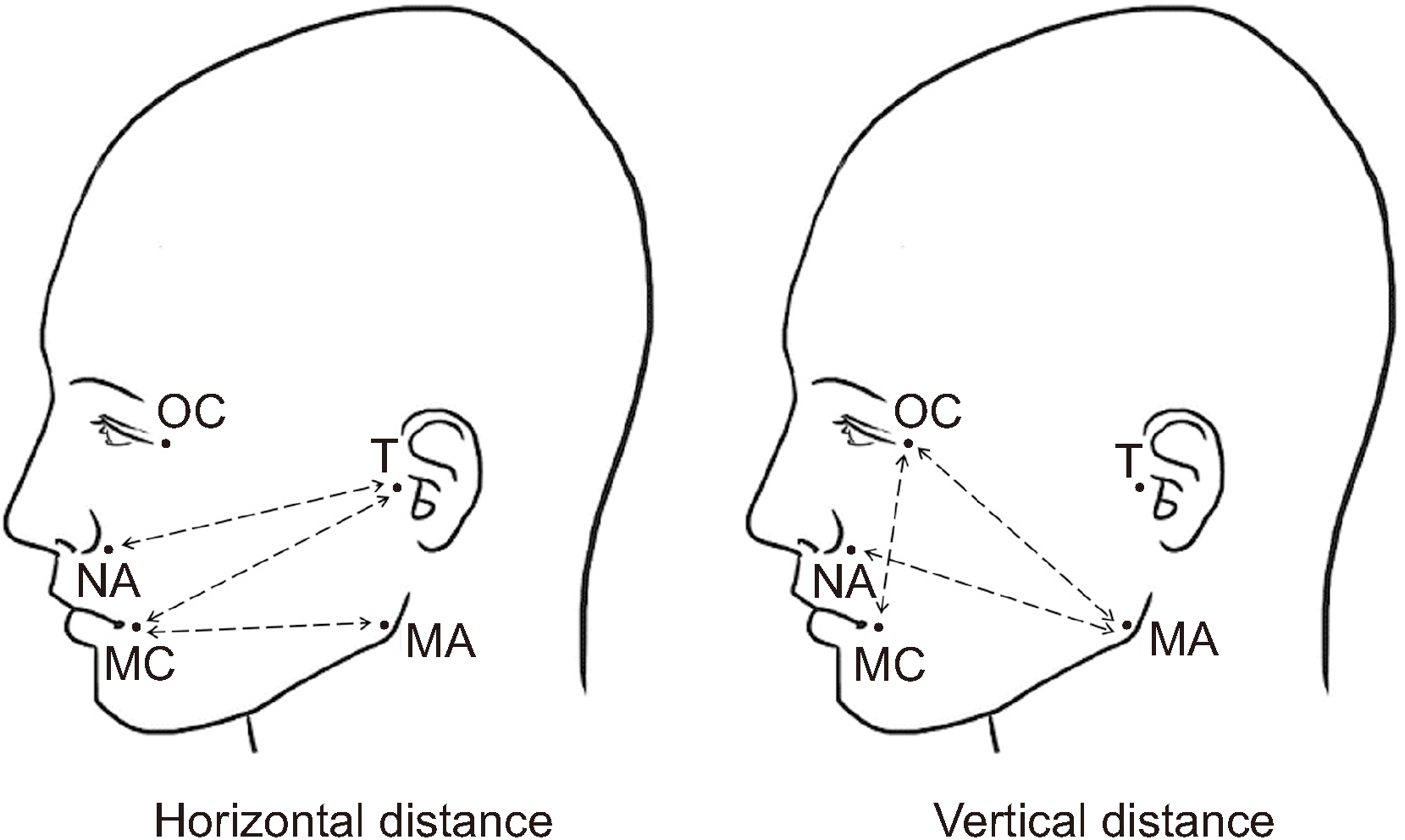 | Fig. 1Measurement points: tragus (T), mouth corner (MC), nasal alar (NA), mandible angle (MA), outer canthus (OC). Six different measurements were made between the landmarks: horizontal distance (T-MC, T-NA, MA-MC), vertical distance (MA-NA, MA-OC, OC-MC). 
|
The degree of pain at T2 and the degree of pain at 10-14 days after surgery (T3) were assessed using the numerical rating scale (NRS). The extent of pain reduction was evaluated by calculating the difference between T2 and T3.
All adverse events that occurred during the clinical trial were included in the safety assessment, recorded in the case report, and their abnormality evaluated. Treatment-emergent adverse events were recorded up to 14 weeks from the time of application of the clinical trial drug. Hyaluronidase is a product already licensed for use; therefore, no special side effects or safety risks were expected. However, if an abnormal reaction did occur, then the study was discontinued and treatment of the patient was considered top priority. An explanation was provided to every patient whenever there was a question, and the trial was stopped immediately if any patient decided to no longer participate.
1. Details regarding clinical schedules and methodology
1) Assessment 1 (baseline screening)
For patients who provided written consent, the demographic information was collected and whether bone grafts were used was recorded. Registered subjects were numbered sequentially, starting from R01, and patients were assigned to the test group and control group. The randomization code was generated by a statistician using a block randomization method and a computer program; the contents were enclosed in an envelope and transmitted to the person in charge.
2) Assessment 2 (day of surgery)
Patients enrolled in the study underwent alveolar bone augmentation with or without hyaluronidase. The length of the line connecting a specific landmark on the face to the surgical site was measured using a flexible ruler.
3) Assessment 3 (2-4 days after surgery)
The occurrence of complications such as abnormal pain, wound healing, and edema at the surgical site, were investigated. The length of the line connecting a specific landmark on the face to the surgical site was measured using a flexible ruler. The degree of pain was assessed using the NRS.
4) Assessment 4 (10-14 days after surgery)
The presence or absence of side effects was evaluated. In addition, the degree of pain was assessed using the NRS.
2. Statistical analysis
Data associated with the test group or control group, consent acquisition date, name, initials, registration number, sex, date of birth, dates of the first, second, third, and fourth visits, edema, and pain were organized and saved using Microsoft Excel 2010 (Microsoft, Redmond, WA, USA). The Shapiro–Wilk test was used to evaluate the data distribution. Because the data followed a non-normal distribution, differences between groups were assessed using the nonparametric Mann–Whitney U test. Statistical analyses were performed using IBM SPSS Statistics (ver. 20; IBM, Armonk, NY, USA). Descriptive statistics, including the median values and interquartile ranges (IQR), were determined for all variables of the control and test groups. The criterion for statistical significance was P<0.05.
Go to :

IV. Discussion
HA is a major carbohydrate component that constitutes the structural framework of the interstitial barrier of various tissues in the body. HA is an anionic, non-sulfated glycosaminoglycan. As a molecule constituting the extracellular matrix, HA has unique hygroscopic, rheological, and viscoelastic properties. Edema is a common complication after surgery. The strong hydrophilic and hydration activities of HA prevent extravascular fluid from returning to the lymphatic system, thus contributing to the continued edematous condition
7. Various attempts have been made to reduce edema after swelling because edema early during the healing phase can cause severe pain and dehiscence of the surgical site, thus delaying healing.
Hyaluronidase was first introduced by Duran-Reynals
12; at that time, hyaluronidase was thought to reduce tissue impermeability, therefore, it was called “spreading/permeability factor.” Later, the substance was discovered to selectively hydrolyze HA and loosen the extracellular matrix. Therefore, it was called “hyaluronidase”
12,13. As an HA-metabolizing enzyme, hyaluronidase breaks the 1,4-glucosaminidic bond between the C1 and glucosamine moiety of HA, the ground substance of connective tissue, and C4 of glucuronic acid
8. Consequently, the local intracellular permeability of peripheral lymphatic channels and capillaries increases and viscosity decreases. This spreads the fluid inside the interstitial space and helps reduce edema. Due to this diffusion capacity, when administered adjunctively, hyaluronidase helps increase the analgesic efficacy of local anesthetics
10.
The anti-inflammatory and anti-edema effects of topically applied hyaluronidase have been previously described. When topically administered, increased numbers of leukocytes, mononuclear cells, and neutrophils were observed, and its rolling adhesion capacity was increased. Reportedly, the levels of tumor necrosis factor-α, interleukin-8, and leukotriene C4 at the inflammation site were also reduced
14. Because HA is a type of glycosaminoglycan with a half-life of approximately 20 hours, the connective tissue is reportedly restored to its original structure within approximately 2 days after administration, therefore, there is no permanent site change
15.
GBR surgery can be used to reconstruct hard tissue into an ideal form before implant placement. Tension-free primary closure is a prerequisite for successful GBR surgery results. If a periosteal releasing incision is created to obtain primary wound closure, then postoperative edema due to the accumulation of serous fluid in the interstitial space in response to surgical trauma is inevitable. Postoperative edema is the most common complication and can lead to increased pain and dehiscence of the surgical site. Various methods to reduce edema have been introduced, however, the optimal approach to reduce edema has not yet been identified. In addition, postoperative inflammatory reactions reach a maximum on the second day after surgery and gradually disappear over the course of the following week. Eliminating other factors that may affect early wound healing is important because this may improve the quality of life during the first week after surgery and the surgical outcome
16.
For several years, hyaluronidase has been used in various fields of medicine to minimize postoperative edema. Han et al.
17 reported injecting hyaluronidase after hematoma and fibrosis due to facial trauma, which resulted in complete resolution of the hematoma, reduced fibrosis, and alleviation of pain. Because nasal bone fractures require surgery after edema is reduced, a considerable amount of time is required between the accident and surgery. Kim et al.
18 applied hyaluronidase to reduce edema before nasal bone fracture surgery during a case-control study of 181 patients and showed that when hyaluronidase was applied, a mean of 3 days from trauma to surgery was required and a mean of 8.6 days was required for the control group. Furthermore, a significant reduction in latency due to the edema-reducing effect of hyaluronidase was reported
18. The effect of hyaluronidase on postsurgical edema reduction has been well-demonstrated in animal studies. For example, Nekoroski et al.
9 demonstrated the diffusion activity of accumulated postsurgical edematous fluid as a result of injecting recombinant human hyaluronidase sustained-release gel in mice with lymphedema.
Among the various methods used to reduce edema after surgery, corticosteroids inhibit phospholipase A2 and lower the activation of the arachidonic acid pathway. Corticosteroids have an excellent anti-edema effect but also reduce leukocyte chemotaxis, fibroblast migration, and collagen synthesis, thereby slowing healing and suppressing the immune response. Consequently, corticosteroids are not the first choice of treatment after surgery. Koç and Er
19 induced traumatic edema in rats and compared the anti-edema effect of the topical administration of hyaluronidase and corticosteroid and reported that hyaluronidase can be used as a substitute for dexamethasone because it reduces edema more effectively.
In the field of dentistry, hyaluronidase has been used in only a few clinical studies to reduce postoperative edema. In the present study, whether hyaluronidase can be used as an adjunct in dental bone surgery to improve postoperative satisfaction with minimal side effects was evaluated; significant improvements in postoperative swelling after directly injecting hyaluronidase in the surgical site were observed.
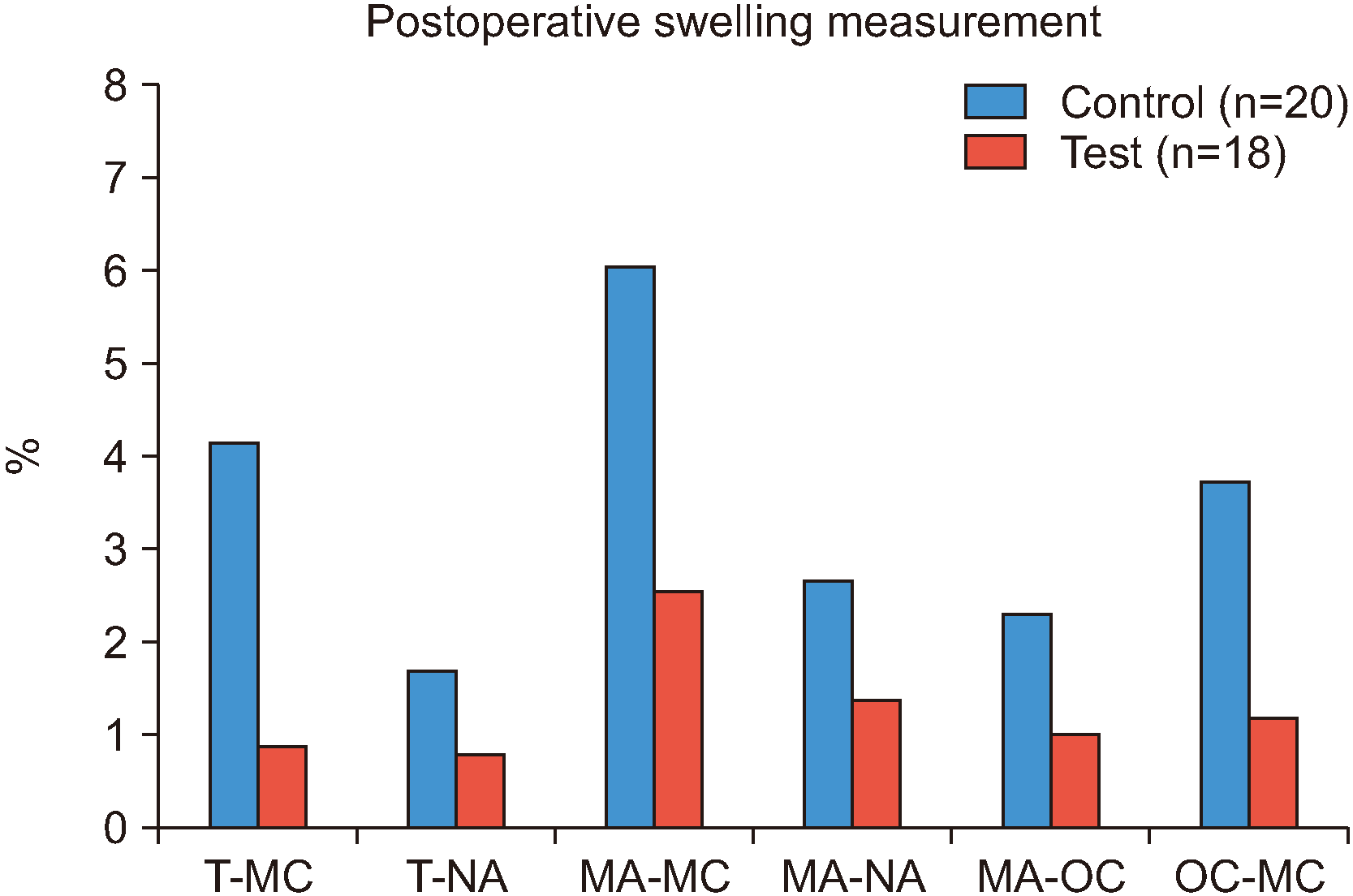
The degree of edema in the oral and maxillofacial regions is generally assessed by two measurements. In the present study, four additional values were measured
20,21 and comparison of the two groups revealed that not all measurements were significantly different. However, the degree of edema in the test group tended to be lower than in the control group. Significant edema reduction was observed in the T-MC (horizontal;
P=0.001) and OC-MC (vertical;
P=0.013) values. Because the mean age of the test group was 4.5 years younger than in the control group, a study with a larger sample size is needed to investigate the effect of age.
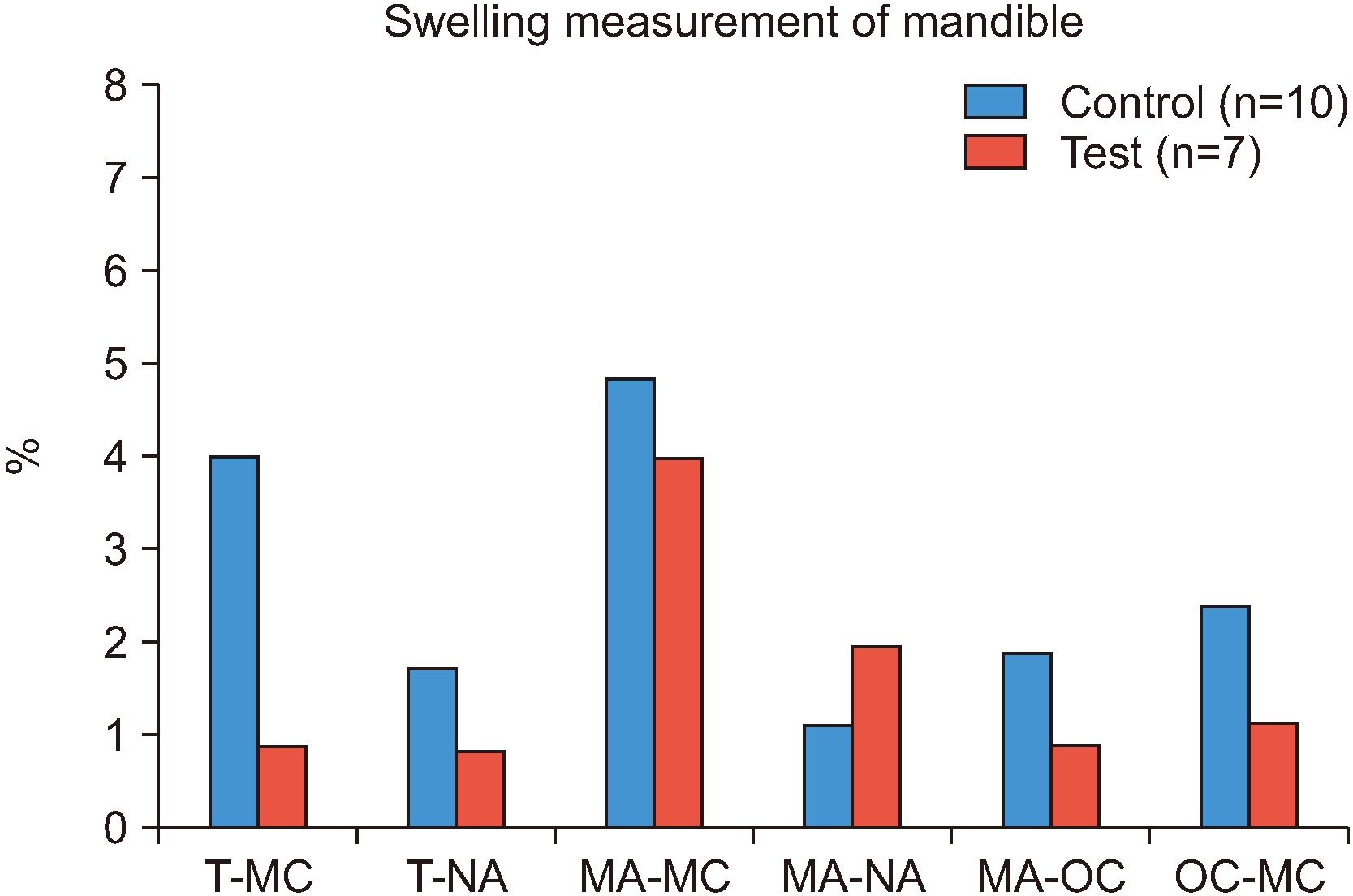
When comparing the maxilla and mandible of the test and control groups, three measurements of the maxilla and one measurement of the mandible showed significant edema reduction. Specifically, in the maxilla, the T-MC value (P=0.034), which is a horizontal measurement, and the MA-NA (P=0.037) and OC-MC (P=0.035) values, which are vertical measurements, showed statistically significant edema reduction. Only one T-MC value (horizontal measurement) of the mandible (P=0.019) was statistically significant. These findings indicate that edema reduction through the local injection of hyaluronidase is more effective in the maxilla than in the mandible.
Hyaluronidase was reported to have an analgesic effect in several studies
10,8. However, in the present study, significant difference was not observed between the test and control groups. Patients were instructed to use painkillers before and after surgery. We suggest an additional method should be devised to control the masking effect of painkillers to accurately determine the degree of hyaluronidase-induced pain reduction. In the present study, the NRS was used to evaluate the severity of pain. One limitation of the NRS is the degree of pain can be underestimated or overestimated. However, NRS is a reliable method suitable for assessing self-reported pain because it is simple and straightforward
22.
In this prospective study, the effect of the topical application of hyaluronidase during GBR surgery on postoperative edema and pain was evaluated. Although each step in the study was conducted according to the relevant guidelines, standardization of the location and size of the surgical site was not possible. Furthermore, the study was limited by its small sample size. The ability of a local injection of hyaluronidase to reduce edema in the dental field has been assessed in only a few studies. Therefore, further in vivo studies with larger sample size and randomized controlled trials are needed to validate our results.
Despite the need for more research, when applied adjunctively, hyaluronidase has the tendency to reduce edema after surgery without any specific side effects. Therefore, the supplemental use of hyaluronidase in dentistry is considered highly beneficial.
Go to :

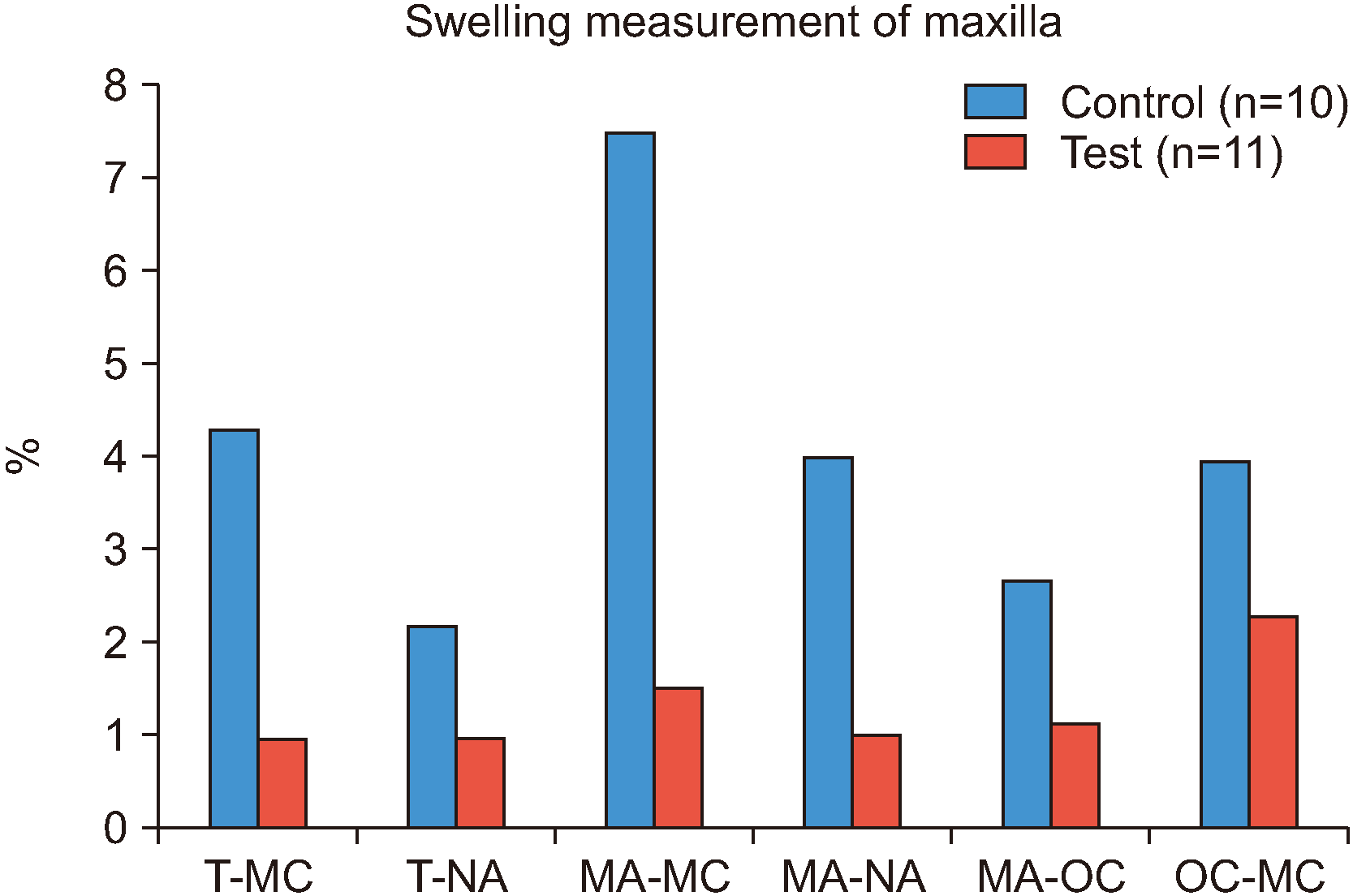




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download