Abstract
The pegylated interferon plus ribavirin combination therapy has been used as the primary treatment for chronic hepatitis C (CHC) but fails to produce a sustained viral response (SVR) in many patients. In recent years, the treatment of CHC has been rapidly changing because of the introduction of direct-acting antivirals (DAAs), which have a high cure rate. However, retreatment of patients after failure of the first DAA therapy is difficult. We report two rare cases of CHC that showed acquired SVR with other DAA combinations after failure to daclatasvir and asunaprevir.
Go to : 
Chronic hepatitis C (CHC) is the most common chronic liver disease in the western world and a major risk factor for liver cirrhosis, portal hypertension, and hepatocellular carcinoma.1 The pegylated interferon and ribavirin combination therapy has long been used as a primary treatment for CHC.2 The genotype 1 HCV infection has been difficult to cure with interferon and ribavirin combination therapy, with cure rates of approximately 50%.3
Treatment regimens of CHC are rapidly changing because of the introduction of direct-acting antivirals (DAAs), which are extremely potent, well tolerated, and have fewer adverse reactions compared to the pegylated interferon and ribavirin combination therapy. Currently, there are several DAAs approved to treat CHC, depending on the HCV genotype.4
Ombitasvir/paritaprevir/ritonavir in combination with dasabuvir (OPrD) is a fixed-dose combination of three DAAs, ritonavir- boosted paritaprevir, ombitasvir, and dasabuvir. A 12-week regimen with or without ribavirin to treat the genotype 1 HCV infection was approved by the US Food and Drug Administration in 2014 and showed cure rates >95% in clinical trials.5,6 Therefore, OPrD has been recommended to treat the genotype 1 HCV infection by both American and European societies of the study of liver diseases.7,8
Glecaprevir/pibrentasvir (GP) is a fixed-dose combination of two pan-genotypic DAAs. It is indicated for adult patients with chronic genotype 1, 2, 3, 4, 5, or 6 HCV infection with or without compensatory cirrhosis. This includes patients with genotype 1 HCV infection who were previously treated with a regimen of NS5A inhibitor or an NS3/4A protease inhibitor but not with both. The recommended duration was 8, 12, or 16 weeks, depending on the patient's prior treatment experience, genotype, and cirrhosis status.9,10
Retreatment of patients after failure of the first DAA therapy remains challenging. However, a recent study showed that retreatment of patients after failure of the first NS5A-based regimen is effective.11 In addition, the present cases show that retreatment of a patient after failure of the first NS5A-based regimen is effective. Therefore, we report two cases of 53- and 58-year-old women who were successfully retreated with OPrD and GP after failure to achieve SVR with daclatasvir and asunaprevir.
Go to : 
The patient was a 53-year-old woman with liver cirrhosis who showed a null response to prior pegylated interferon and ribavirin combination therapy. She visited the hospital for elevated liver enzyme levels. Blood tests showed a white blood cell count of 3,840/mm3, hemoglobin level of 10.9 g/dL, platelet count of 79,000/mm3, AST of 141 U/L, ALT of 134 U/L, total protein of 7.3 g/dL, total albumin of 3.7 g/dL, PT of 14.6 sec, and total bilirubin of 1.3 mg/dL. Abdominal ultrasonography revealed liver cirrhosis without ascites. She was positive for anti-HCV, HCV RNA level of 122,000 IU/mL, genotype 1b, no baseline NS5A or NS3 resistance-associated variants (RAVs) and showed 100% compliance with daclatasvir and asunaprevir treatment.
During treatment with daclatasvir 60 mg once daily and asunaprevir 100 mg twice daily, the patient experienced rapid viral decline and achieved undetectable levels of HCV RNA in week 4. Treatment with daclatasvir and asunaprevir lasted up to 24 weeks and levels of HCV RNA had been undetectable. But HCV RNA in post-treatment week 4 was 597,000 IU/mL and it was determined that relapse occurred. Treatment with daclatasvir and asunaprevir for 24 weeks failed because of the appearance of mutations in the NS5A region of the HCV genome. Both L31M and Y93H mutants were detected.
The patient was retreated with ombitasvir 25 mg/paritaprevir 150 mg/ritonavir 100 mg once daily administered in combination with dasabuvir 250 mg twice daily administered orally for 16 weeks. After 4 weeks of therapy, the level of HCV RNA decreased rapidly to 20 IU/mL, and levels of AST and ALT were normalized. Retreatment with OPrD achieved undetectable levels of HCV RNA in week 8. HCV RNA was not detected, and serum AST and ALT levels remained within normal limits throughout the treatment and follow-up periods. The patient achieved SVR in both post-retreatment weeks 8 and 24 (Fig. 1).
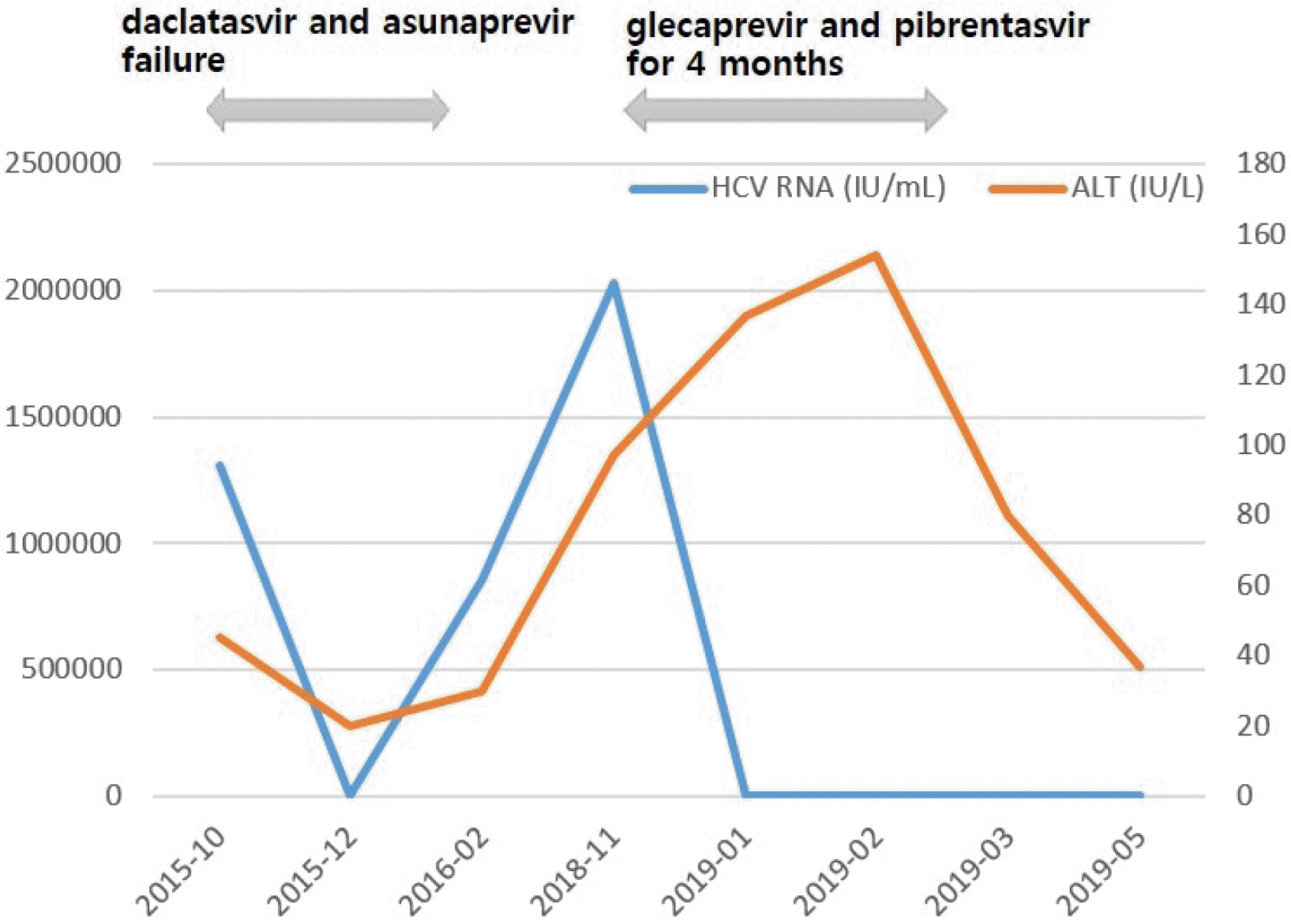
A 58-year-old woman with fatty liver disease visited the hospital because of elevated liver enzyme levels. Blood tests showed a white blood cell count of 4,550/mm³, hemoglobin level of 13.1 g/dL, platelet count of 172,000/mm3, AST of 217 U/L, ALT of 97 U/L, total protein of 8.4 g/dL, total albumin of 3.9 g/dL, PT of 14.3 s, and total bilirubin of 1.0 mg/dL. Abdominal ultrasonography revealed a mild fatty liver. She had positive anti-HCV, HCV RNA levels of 2,030,000 IU/mL, and genotype 1b. She had a previous medical history of treatment failure with pegylated interferon and subsequent DAAs with daclatasvir and asunaprevir for 6 months at another hospital. The mutations in the NS5A region of the HCV genome were L31 negative and Y93 positive.
The patient was retreated with glecaprevir (300 mg) and pibrentasvir (120 mg) once daily administered orally for 16 weeks. After 7 weeks of therapy, HCV RNA was not detected; however, serum levels of AST and ALT were not normalized. At the 1-month follow-up after a total of 4 months of treatment, HCV RNA was still undetected, and liver enzymes were decreased. The patient achieved SVR in both retreatment weeks 12 and 24 (Fig. 2).
Go to : 
Since 2015, treatment with daclatasvir and asunaprevir achieved high rates of SVR (82-91%) in HCV genotype 1b-infected patients without NS5A RAVs and with or without NS3 RAVs at baseline.12 These cases demonstrated that SVR 24 can be achieved with OPrD or GP in cases of treatment failure with previous antiviral therapy with daclatasvir and asunaprevir, even when the retreatment with NS5A containing regimen.
Current recommendation for retreatment for a DAA failure mentioned that clinician should consider several predictive factors such as type of previous drug, HCV genotype, presence of cirrhosis, and RAS before selection of the adequate regimen. It is recommended that patients with NS5A RAVs but without NS3 RAVs receive a sofosbuvir-based regimen with another DAAS, ideally with no cross-resistance with the drug already administerd.7,8 According to the guidelines of Korean Association for the Study of the Liver, several combination therapies for genotype 1 HCV are recommended if DAA treatment including NS5A inhibitor fails. Then, sofosbuvir/velpatasvir/voxilaprevir, sofosbuvir plus elbasvir/grazoprevir plus ribavirin, sofosbuvir plus OPrD, GP are mentioned to use.13 Current American Association for the Study of Liver Disease guidance mentioned that in NS5A inhibitor DAA-experienced genotype 1 patients, sofosbuvir/velpatasvir/voxilaprevir, or GP could be used.14 However, these clinical guideline/ guidance also mentioned that GP is not indicated to the patient exposed to both NS3/4 protease inhibitor and NS5A inhibitor. Also, sofosbuvir/velpatasvir/voxilaprevir is not approved in Korea yet, therefore, it is difficult to select the optimal option for retreatment of the patients who experienced daclatasvir (NS5A inhibitor) and asunaprevir (NS3/4 protease inhibitor) right now.
A study from Japan reported that retreatment of ledipasvir/ sofosbuvir for 12 weeks in the HCV genotype 1b patients who failed in daclatasvir and asunaprevir showed SVR rate 70%, which is suboptimal result.15 In SAPPHIRE-II trial, retreatment of OPrD plus ribavirin in the patients with HCV genotype 1b who had failed with pegylated interferon and ribavirin showed the SVR rate as high as 97%. One of the two patients among failures had RAV present at time of relapse had Y56H and D168A in NS3, Y93H in NS5A, and C316N and S556G in NS5B.16 An analysis of HCV genotype 1b RAVs in Japanese patients who treated with OPrD presented that among patients with Y93H comprising <1%, 1 to 40%, or >40% of their preexisting viral population, SVR rate were >99%, 93%, and 76%, respectively.17 Therefore, although we experienced the success in the retreatment by OPrD in the patient who failed in daclatasvir and asunaprevir and acquired emergent Y93H RAV in Case 1, it is prudent to explain sufficiently the possibility of failure in case that Y93H is dominant in the viral population in the host before treatment decision.
There are some reports dealing with retreatment of GP in HCV-infected patients with prior DAA-experience. According to Tojima et al.,18 all 21 patients who experienced treatment failure by daclatasvir and asunaprevir had SVR by GP as 2nd, 3rd, or 4th line treatment. On the other hand, Kumada et al.19 presented that two of 30 (6.7%) genotype 1b-infected patients had previously received daclatasvir and asunaprevir had P32 deletion in NS5A at baseline, and both of these patients experienced virologic failure. P32 deletion in NS5A confers >1,000-fold resistance to pibrentasvir. Therefore, there is possibility that P32 deletion in NS5A may be related to retreatment failure by GP among the patients experienced daclatasvir and asunaprevir. So, it is relevant to inform the patients who are going to have retreatment with GP of the possibility of failure due to RAV.
If only considering the antiviral efficacy in retreatment in the patients who failed in daclatasvir and asunaprevir, it is best option to wait and see the patients without treatment until sofosbuvir/velpatasvir/voxilaprevir is introduced in clinical practice. Meanwhile, the patients with advanced liver fibrosis and persistent necroinflammation related to progression of liver disease may be treated by other NS5A inhibitor containing regimens such as OPrD or GP after discussion of risk and benefit with the patients.
This report showed that the patients with HCV genotype 1b who failed in daclatasvir and asunaprevir may get help with OPrD or GP as 2nd line treatment. However, physician also should be careful at the patient selection for urgent treatment.
Go to : 
REFERENCES
1. Polaris Observatory HCV Collaborators. 2017; Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2:161–176. DOI: 10.1016/S2468-1253(16)30181-9. PMID: 28404132.

2. Feld JJ, Hoofnagle JH. 2005; Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature. 436:967–972. DOI: 10.1038/nature04082. PMID: 16107837.

3. Manns MP, McHutchison JG, Gordon SC, et al. 2001; Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 358:958–965. DOI: 10.1016/S0140-6736(01)06102-5. PMID: 11583749.


4. Feld JJ, Foster GR. 2016; Second generation direct-acting antivirals - do we expect major improvements? J Hepatol. 65 Suppl 1:S130–S142. DOI: 10.1016/j.jhep.2016.07.007. PMID: 27641983.

5. Feld JJ, Kowdley KV, Coakley E, et al. 2014; Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 370:1594–1603. DOI: 10.1056/NEJMoa1315722. PMID: 24720703.


6. Poordad F, Hezode C, Trinh R, et al. 2014; ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 370:1973–1982. DOI: 10.1056/NEJMoa1402869. PMID: 24725237.


7. AASLD/IDSA HCV Guidance Panel. 2015; Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 62:932–954. DOI: 10.1002/hep.27950. PMID: 26111063.

8. European Association for the Study of the Liver. 2018; EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 69:461–511. DOI: 10.1016/j.jhep.2018.03.026. PMID: 29650333.
9. Forns X, Lee SS, Valdes J, et al. 2017; Glecaprevir plus pibrentasvir for chronic hepatitis C virus genotype 1, 2, 4, 5, or 6 infection in adults with compensated cirrhosis (EXPEDITION-1): a single-arm, open-label, multicentre phase 3 trial. Lancet Infect Dis. 17:1062–1068. DOI: 10.1016/S1473-3099(17)30496-6. PMID: 28818546.


10. Zeuzem S, Foster GR, Wang S, et al. 2018; Glecaprevir-pibrentasvir for 8 or 12 weeks in HCV genotype 1 or 3 infection. N Engl J Med. 378:354–369. DOI: 10.1056/NEJMoa1702417. PMID: 29365309.


11. Halfon P, Scholtès C, Izopet J, et al. 2018; Retreatment with direct-acting antivirals of genotypes 1-3-4 hepatitis C patients who failed an anti-NS5A regimen in real world. J Hepatol. 68:595–597. DOI: 10.1016/j.jhep.2017.09.019. PMID: 28987520.


12. Manns M, Pol S, Jacobson IM, et al. 2014; All-oral daclatasvir plus asunaprevir for hepatitis C virus genotype 1b: a multinational, phase 3, multicohort study. Lancet. 384:1597–1605. DOI: 10.1016/S0140-6736(14)61059-X. PMID: 25078304.


13. Korean Association for the Study of the Liver (KASL). 2018; 2017 KASL clinical practice guidelines management of hepatitis C: treatment of chronic hepatitis C. Clin Mol Hepatol. 24:169–229. DOI: 10.3350/cmh.2018.1004. PMID: 30092624. PMCID: PMC6166104.


14. NS5A inhibitor DAA-experienced, genotype 1 patients. [Internet]. 2021. Jan. 21. American Associations for the Study of Liver Diseases (AASLD);Alexandria (VA): cited 2021 Jan 28. Available from https://www.hcvguidelines.org/treatment-experienced/multiple-daa-failure.
15. Akuta N, Sezaki H, Suzuki F, et al. 2017; Ledipasvir plus sofosbuvir as salvage therapy for HCV genotype 1 failures to prior NS5A inhibitors regimens. J Med Virol. 89:1248–1254. DOI: 10.1002/jmv.24767. PMID: 28079269.


16. Zeuzem S, Jacobson IM, Baykal T, et al. 2014; Retreatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 370:1604–1614. DOI: 10.1056/NEJMoa1401561. PMID: 24720679.


17. Krishnan P, Schnell G, Tripathi R, et al. 2015; Analysis of hepatitis C virus genotype 1b resistance variants in Japanese patients treated with paritaprevir-ritonavir and ombitasvir. Antimicrob Agents Chemother. 60:1106–1113. DOI: 10.1128/AAC.02606-15. PMID: 26643326. PMCID: PMC4750684.


18. Tojima H, Kakizaki S, Takakusagi S, et al. 2020; Favorable outcome of retreatment by direct-acting antivirals for hepatitis C patients with daclatasvir plus asunaprevir combination therapy failure. Hepatol Res. 50:303–312. DOI: 10.1111/hepr.13462. PMID: 31750974.
19. Kumada H, Watanabe T, Suzuki F, et al. 2018; Efficacy and safety of glecaprevir/pibrentasvir in HCV-infected Japanese patients with prior DAA experience, severe renal impairment, or genotype 3 infection. J Gastroenterol. 53:566–575. DOI: 10.1007/s00535-017-1396-0. PMID: 29052790. PMCID: PMC5866827.


Go to : 




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download