A-50-year-old man with no significant/relevant medical history was admitted to Wonkwang University Hospital with sudden- onset epigastric abdominal pain, which began after performing push-ups, but no trauma. Upon admission, a physical examination revealed a mildly distended abdomen with epigastric tenderness. The patient’s blood pressure was 50/40 mmHg, with a heart rate of 107 beats/min. Rapid fluid resuscitation restored the patient’s circulation status. After the blood pressure was stabilized, contrast-enhanced CT was performed. Contrast extravasation of the inferior pancreaticoduodenal artery (PDA) was observed, which appeared to be due to the aneurysmal sac (
Fig. 1A). A large-volume hemoperitoneum in the right anterior pararenal space and perihepatic space was evident (
Fig. 1A, B). A mild hypodense lesion at the head of the pancreas, suggesting acute pancreatitis, was also observed (
Fig. 1C). Laboratory investigations performed 2 hours after admission revealed a hemoglobin level of 9.1 g/dL. Emergency angiography was performed immediately to determine the source of bleeding and control it. A 6 Fr sheath was placed into the right common femoral artery, and a right heart catheter and microcatheter were then used to perform the celiac, gastroduodenal, and pancreaticoduodenal arteriographies. The angiographic findings revealed severe CAS and compensation of hepatic arterial blood flow from the PDA (
Fig. 2A). In addition, extravasation of the contrast material in the superior anterior PDA was detected (
Fig. 2B). After selecting superior anterior PDA, stent-graft insertion was attempted, but it was difficult to proceed due to the vascular tortuosity. Collateral circulation appeared to be sufficient on superior mesenteric arteriography. For that reason, angioembolization was performed using a mixture of N-butyl cyanoacrylate and lipiodol (1:4). No procedure-related complications were encountered. The patient was hospitalized in the intensive care unit and followed-up carefully because of the large volume of hemoperitoneum and decreased hemoglobin levels. Octreotide acetate administration and fasting with total parenteral nutrition were administered to prevent the exacerbation of acute pancreatitis. Two days later, abdominal CT angiography was repeated, which revealed a slight decrease in the hemoperitoneum in the anterior pararenal space (
Fig. 3A). CAS was also observed (
Fig. 3B). A fever of 38.4℃ was recorded on day 18 of hospitalization. Percutaneous drainage of the anterior pararenal hematoma was performed under the suspicion of infection in the hematoma. Nevertheless, the fever and leukocytosis persisted, and a pre-rectal abscess was found on CT performed under suspicion of other infection foci. On day 37 of hospitalization, laparoscopic irrigation and drainage were performed. Subsequently, the hematoma improved gradually, but the serum levels of amylase and lipase increased gradually to 195 IU/L and 450 IU/L, respectively. ERCP was performed under the suspicion of acute necrotizing pancreatitis on MRCP (
Fig. 4A). There was no significant leakage or dilatation in the main pancreatic duct. On the other hand, an examination of amylase and lipase in the drainage tube placed after surgery could not exclude continuous pancreatic juice leakage. Therefore, plastic stenting was performed because of the concerns about acute pancreatitis exacerbation and the potential worsening of the intraperitoneal infection (
Fig. 4B). The patient’s laboratory findings stabilized, and he was discharged on day 62 of hospitalization without any related symptoms. A CT scan performed 6 months later revealed marked improvement in the organizing hematoma with adjacent fibrotic changes (
Fig. 5).
 | Fig. 1Initial computed tomography (CT) performed in the emergency department. (A) Axial CT demonstrating contrast extravasation of the inferior pancreaticoduodenal artery (PDA) (arrow), which appears to be due to an aneurysmal sac, and large acute hematoma in the right anterior pararenal space and perihepatic space are shown. (B) CT revealing a mild hypodense lesion at the pancreas head, suggesting acute pancreatitis (arrow). (C) Maximum intensity projection image revealing aneurysmal dilatation of the inferior PDA (arrow). 
|
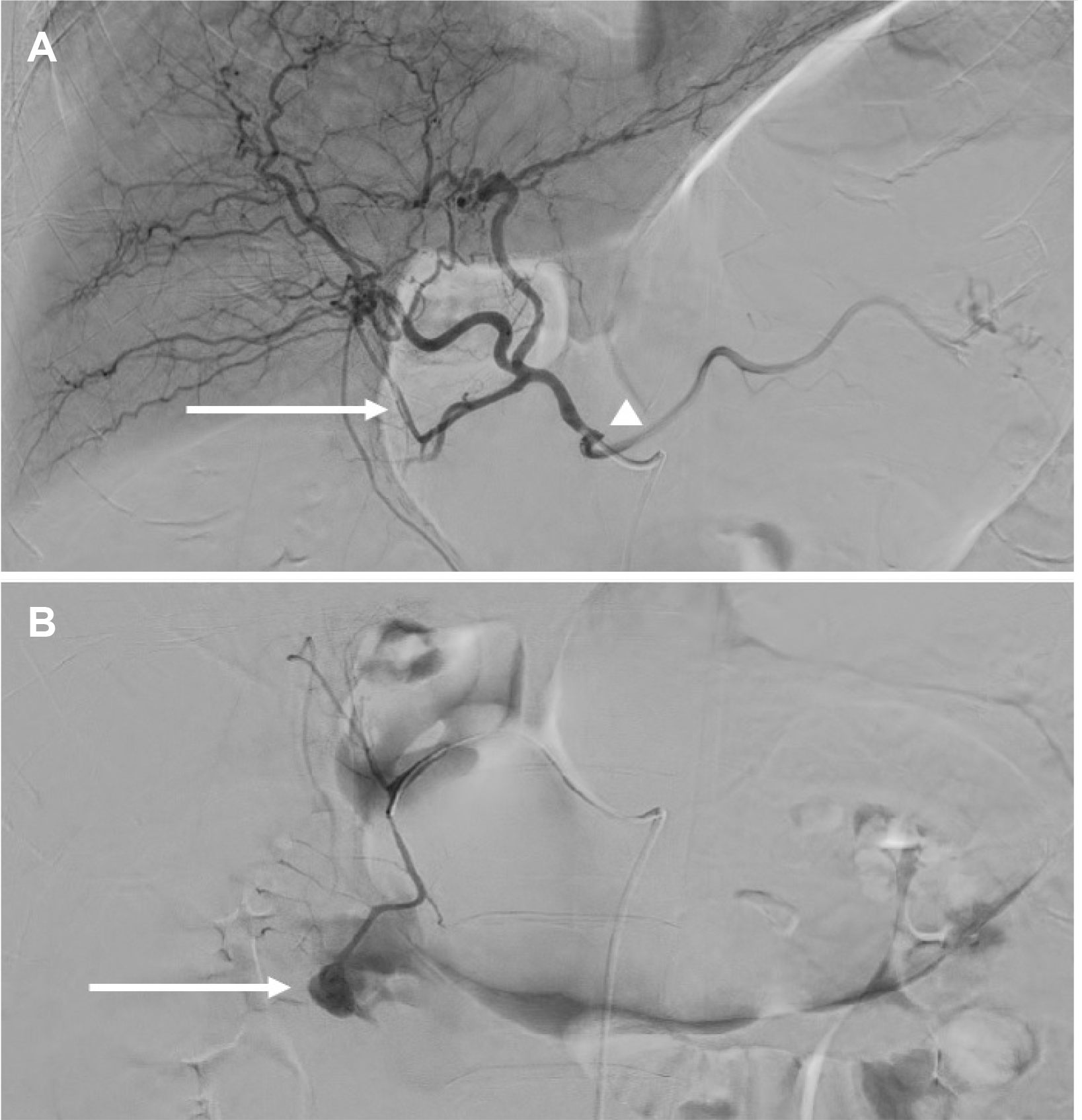 | Fig. 2Arteriography after initial resuscitation. (A) Celiac artery stenosis (CAS) (arrowhead) and hepatic arterial blood flow from the pancreaticoduodenal artery (PDA) are shown (arrow). (B) Large pseudoaneurysm and extravasation in the superior anterior PDA were detected (arrow). 
|
 | Fig. 3Vascular-aorta computed tomography angiography after 2 days of transarterial embolization. (A) Coronal image revealing slightly decreased size of hematoma in the anterior pararenal space. (B) Celiac artery stenosis is also apparent (arrow). 
|
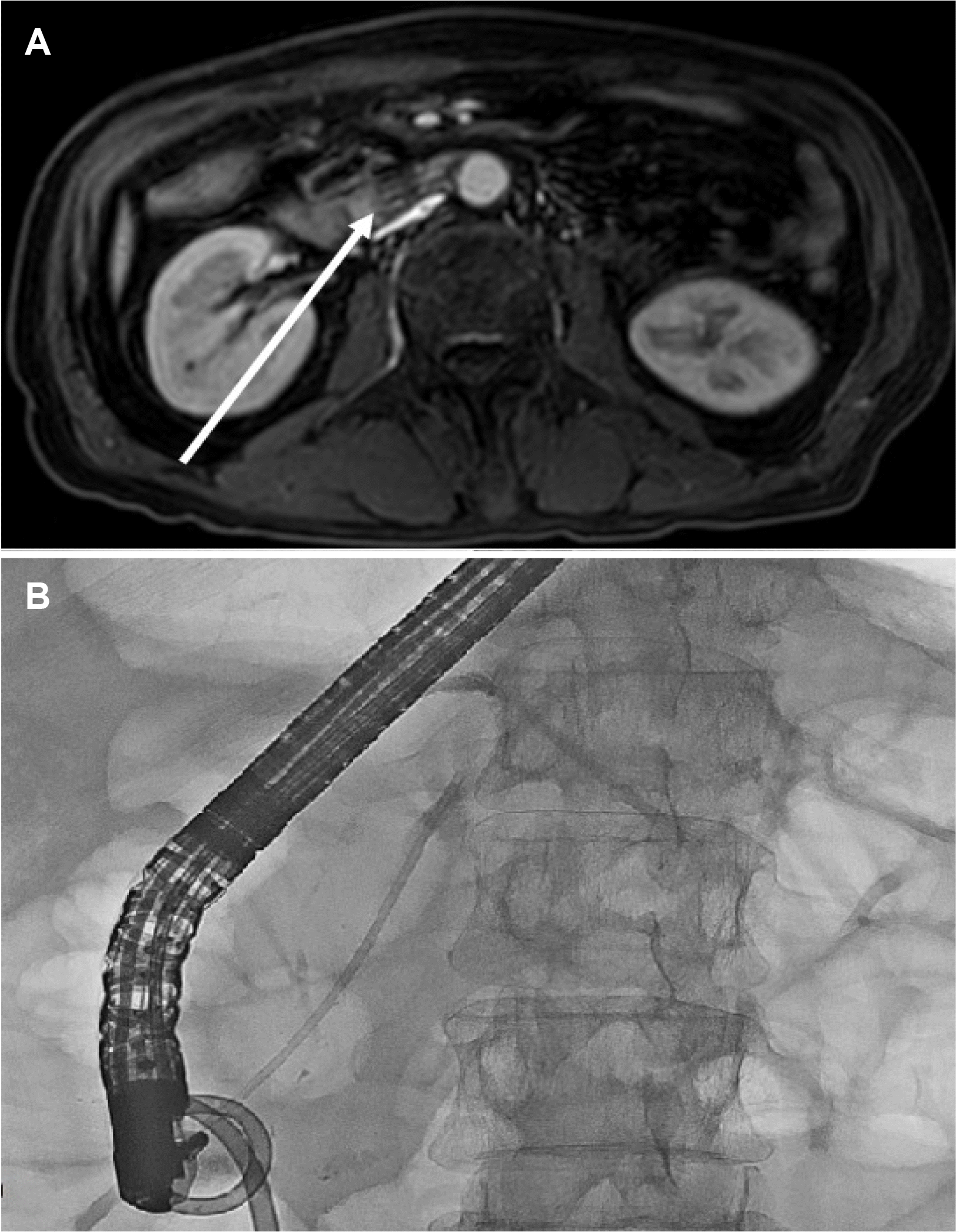 | Fig. 4Magnetic resonance imaging and endoscopic retrograde cholangiopancreatography (ERCP) performed to determine the etiology of the elevated serum amylase and lipase and treat pancreatitis (A) increased signal intensity in the T1-weighted image at the head of the pancreas (arrow). (B) ERCP with plastic stenting in the pancreatic duct was performed. 
|
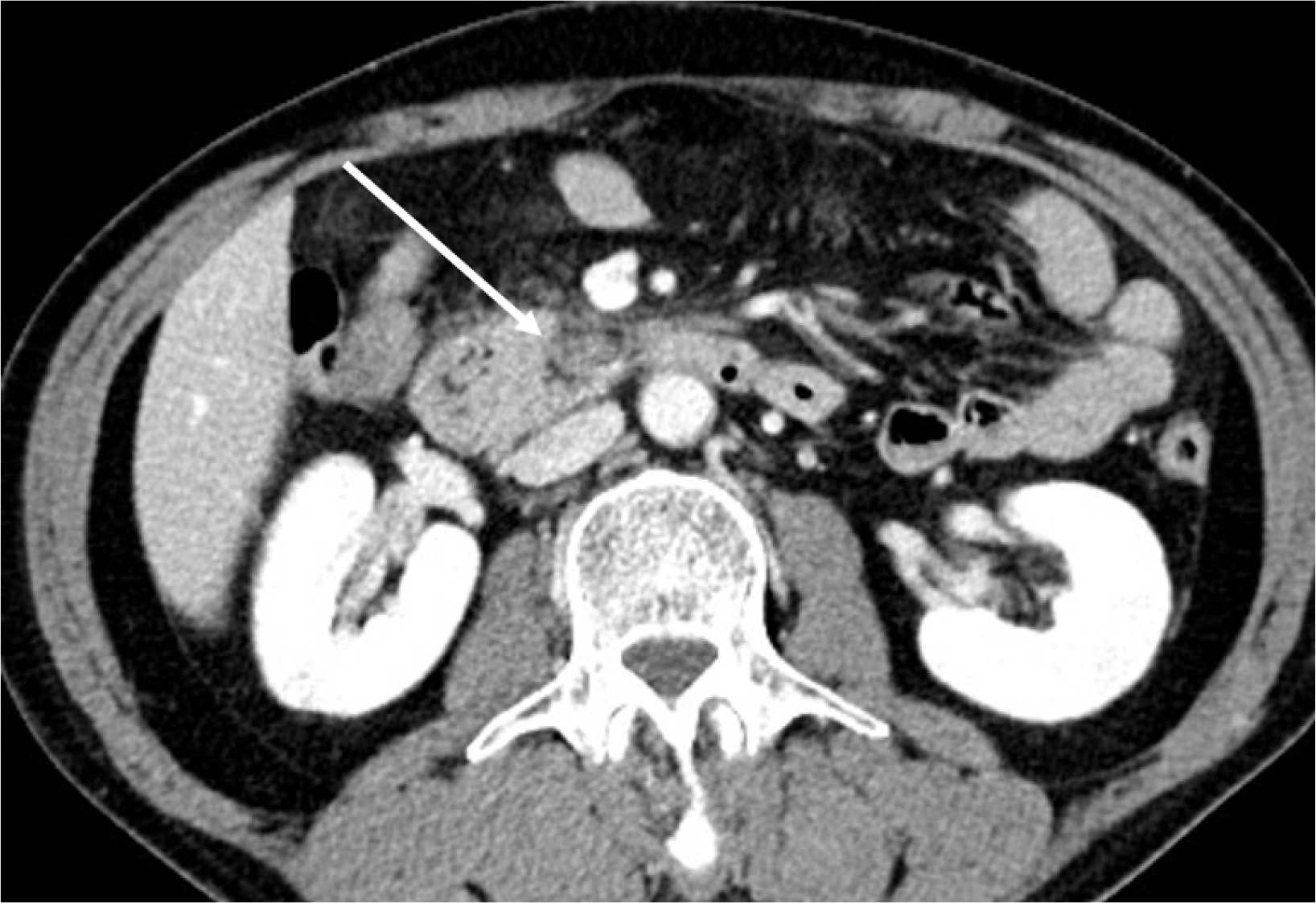 | Fig. 5Computed tomography performed 6 months after discharge. Marked decreased size of the previously noted mass-like lesion with mild haziness in the retromesenteric space, suggesting an improving state of organizing hematoma with adjacent fibrotic changes (arrow). 
|








 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download