INTRODUCTION
Metabolic syndrome is a disease and a risk factor for cardiovascular diseases, including diabetes mellitus (DM), hypertension, dyslipidemia, and central obesity. This is related to insulin resistance. Patients with this condition have a high risk of cardiovascular events.
1,2 Individuals with metabolic syndrome had a significantly higher prevalence of non-alcoholic fatty liver disease (NAFLD) than the controls.
3 Moreover, individual metabolic abnormalities, such as increased waist circumference, impaired fasting glucose/DM, high triglyceride, and low high-density lipoprotein levels, are associated independently with NAFLD.
3 This suggests that many patients with metabolic syndrome are vulnerable to cardiovascular disease and NAFLD, which can progress to hepatic fibrosis. The association between alcohol and metabolic syndrome is complex. High HDL cholesterol levels in alcoholics may reduce the risk of metabolic syndrome, but many people who drink socially often present high blood pressure and glucose levels, high triglyceride levels, and visceral obesity, which may increase the risk of metabolic syndrome. According to meta-analysis, low alcohol intake (≤5 g/day) is associated with a low risk of metabolic syndrome, whereas heavy alcohol intake (≥35 g/day) is associated with a high risk of metabolic syndrome.
4 On the other hand, the risk of aggravating liver disease by viral hepatitis has decreased since the introduction of effective antiviral agents for patients with hepatitis B or C, but the risk of steatohepatitis with metabolic abnormalities remains.
Although physicians often encounter patients with steatohepatitis and metabolic syndrome together in various backgrounds, there is little to improve their liver function. Generally, lifestyle modification, including diet, exercise, and alcohol reduction, is helpful, but most patients find it difficult to adopt a strictly abstinent lifestyle. Many therapeutic drugs for patients with NAFLD have been studied, but very few drugs have improved steatohepatitis in clinical trials.
Biphenyl dimethyl dicarboxylate (BDD) is a hepatotonic agent that reduced hepatocellular damage and fibrosis in a rat model of liver fibrosis induced by carbon tetrachloride.
5 Ursodeoxycholic acid (UDCA) is a type of bile acid that affects cholestatic liver disease, such as primary biliary cholangitis. On the other hand, it is uncertain that this drug is effective for various other liver diseases. In 2004, a study showed that a combination of BDD/UDCA had a greater effect on ALT normalization than a BDD single regimen in patients, among whom viral hepatitis was the dominant cause of chronic liver disease.
6 Nevertheless, there is limited data on the efficacy of this combination for other liver diseases. Therefore, this study evaluated the efficacy of BDD/UDCA in improving necro-inflammation in patients with steatohepatitis related to metabolic syndrome.
SUBJECTS AND METHODS
1. Study design and subjects
This study was a randomized, double-blind, placebo-controlled clinical trial in a single center to evaluate the effects of BDD/UDCA (UDEX®, Pharmbio Korea, Seoul, Korea) on the serum aminotransferase level in patients with chronic liver disease and metabolic syndrome. The inclusion criteria were subjects aged ≥20 years, ALT level ≥60 U/L, and at least one component of metabolic syndrome, such as central obesity (waist circumference ≥90 cm in males and ≥85 cm in females), high blood pressure (systolic blood pressure ≥130 mmHg±diastolic blood pressure ≥85 mmHg or medication history for hypertension), high glucose (fasting glucose ≥100 mg/dL, or medication history for DM), low HDL cholesterol (<40 mg/dL in males, <50 mg/dL in females, or medication history for low HDL cholesterol), and high triglyceride (≥150 mg/dL or medication history for hypertriglyceridemia). They also should have the following findings suggestive of chronic liver disease: a history of elevated AST or ALT over the upper limit of the normal range for more than 6 months from screening, abnormal radiologic findings indicative of chronic liver disease or fatty liver on sonography or CT, or a medical history of hepatotonics for chronic liver disease for more than 30 days. The patients were excluded if they had severe hepatitis (ALT >10 times the upper limit of the normal rage), severe alcoholic hepatitis, acute hepatitis A, uncontrolled acute or chronic hepatitis B (HBV DNA ≥2,000 IU/mL), uncontrolled chronic hepatitis C (positive HCV RNA), other chronic liver diseases (including autoimmune hepatitis, primary biliary cholangitis, primary sclerosing cholangitis, Wilson disease, and hemochromatosis), untreated malignancy, decompensated cirrhosis, drug-induced liver injury, uncontrolled DM (HbA1c >8%), organ or bone-marrow transplantation, and pregnancy.
2. Study protocol
The study subjects were screened for eligibility criteria within 28 days from enrollment and had a 2-week washout period if they had been taking any hepatotonics. The subjects were educated on how to modify their lifestyle to improve metabolic syndrome via weight reduction and exercise. They were allowed to maintain adequate medication for hypertension, DM, or dyslipidemia. The patients were advised to abstain from alcohol. The patients with viral hepatitis B were allowed to keep their antiviral agents.
All the subjects meeting the eligibility criteria were assigned randomly (1:1) to an oral combination pill of BDD 12.5 mg and UDCA 50 mg three times a day or to a placebo for 24 weeks using a stratified randomization table according to the presence of alcohol history (men >40 g/day, female >20 g/day) or viral hepatitis (allocation ratio, four in alcohol [-]/virus [-]: two in alcohol [+]/virus [-]: one in alcohol [-]/virus [+]). Assuming that the proportion of ALT normalization after 24 weeks of treatment was 30% in the placebo group and 65% in the intervention group, a sample size of 35 subjects was calculated in each using a two-sample, two-sided proportion test with a power of 0.8, α-value of 0.05, and follow-up loss of 10%.
7
The intervention and placebo drugs were provided as identical capsules in identical containers labeled with code numbers. The treatment was assigned by pharmacists blinded to the identification of the code for each drug. The patients and investigators were also blinded to the treatment assignment.
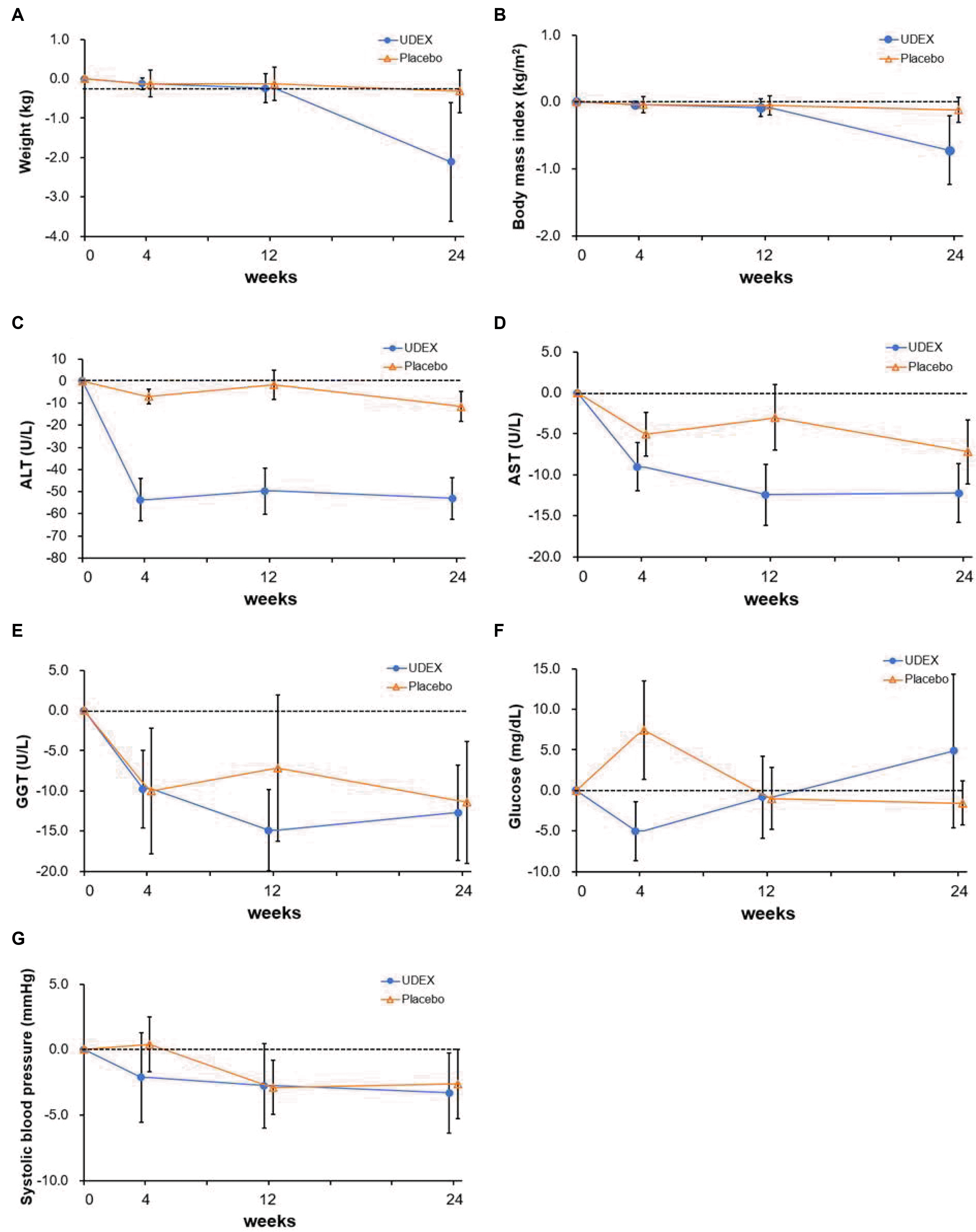
After randomization, subjects returned for study visits at weeks 4, 12, and 24. The vital signs and body weight were measured, and routine biochemical tests were performed at the initial visit and each visit day. Transient elastography (TE) and controlled attenuation parameter (CAP) by FibroScan® (Echosens, Paris, France) were assessed at the initial visit and week 24. The Chronic Liver Disease Questionnaire (CLDQ) was checked to assess the quality of life of the subjects before and after treatment. At each visit, adherence to medication and adverse effects were evaluated. Institutional Review Board in Inje University Haeundae Paik Hospital approved this study (IRB No. HPIRB 2017-11-010).
3. Outcomes
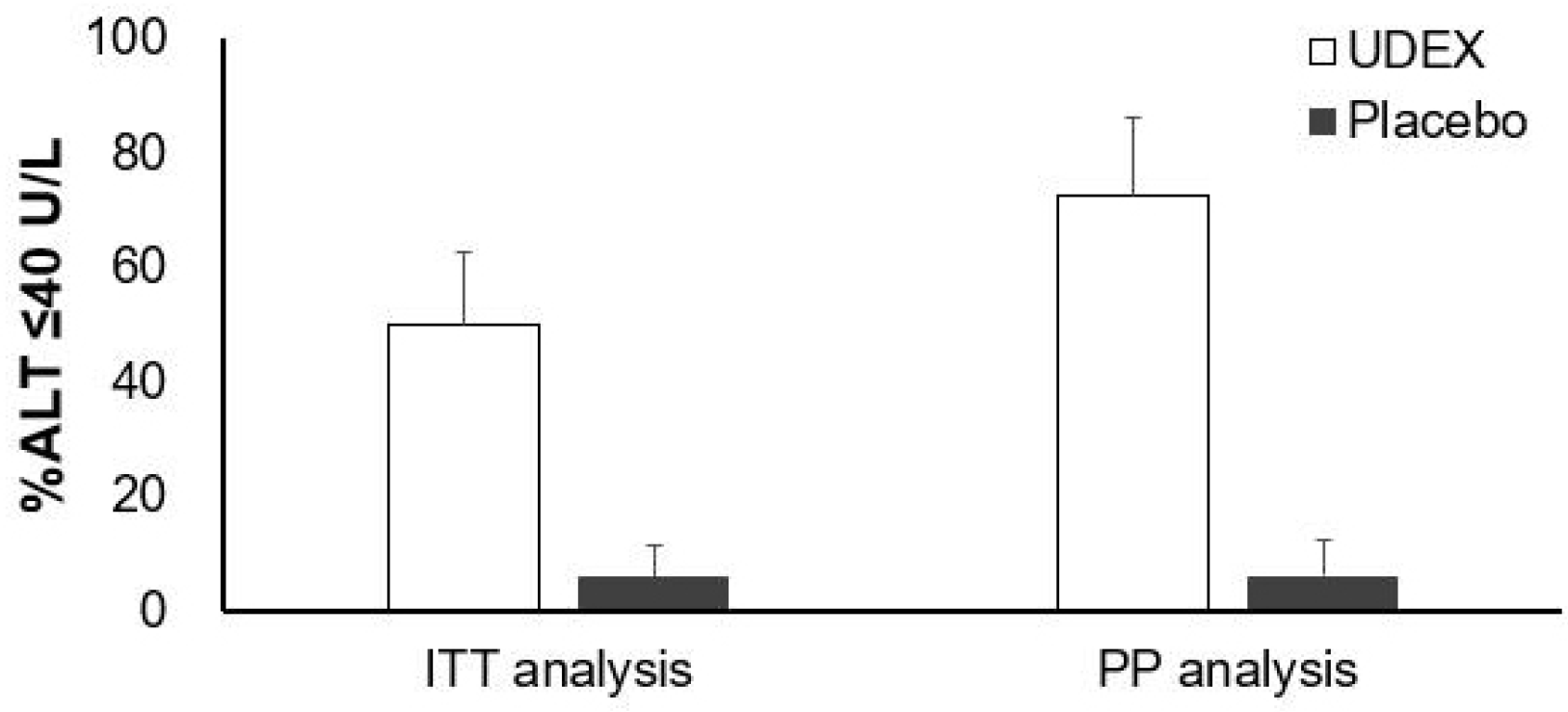
The primary outcome was the proportion of ALT normalization (≤40 U/L) at 24 weeks of administering the study drugs. Secondary outcomes were the change in controlled attenuation parameter and liver stiffness and the change in the score of CLDQ from enrollment to 24 weeks of administration of the study drugs.
4. Statistical analysis
The data are presented as the frequency with a percentage for the categorical variables and as mean±standard deviation and median and interquartile range for the continuous variables. The differences in the characteristics of the study participants across subgroups were compared using a chi-square test or Fisher’s exact test for the categorical variables and an independent test or Mann-Whitney’s U test for the continuous variables as appropriate. A paired t-test or Wilcoxon’s signed-rank test was also used to compare two assessment time points. The Shapiro-Wilk’s test was used to check if the distribution was normal.
A two-way repeated-measures (RM) ANOVA was used to compare the repeated measured numeric variables in groups and within each group. The Bonferroni procedure was applied to post hoc analyses. The last observation carried forward was applied to treat the missing values in intention-to-treat (ITT) analysis. All statistical analyses were carried out using SPSS 24.0 (IBM SPSS Statistics, Chicago, IL, USA), and p-values <0.05 were considered significant.
DISCUSSION
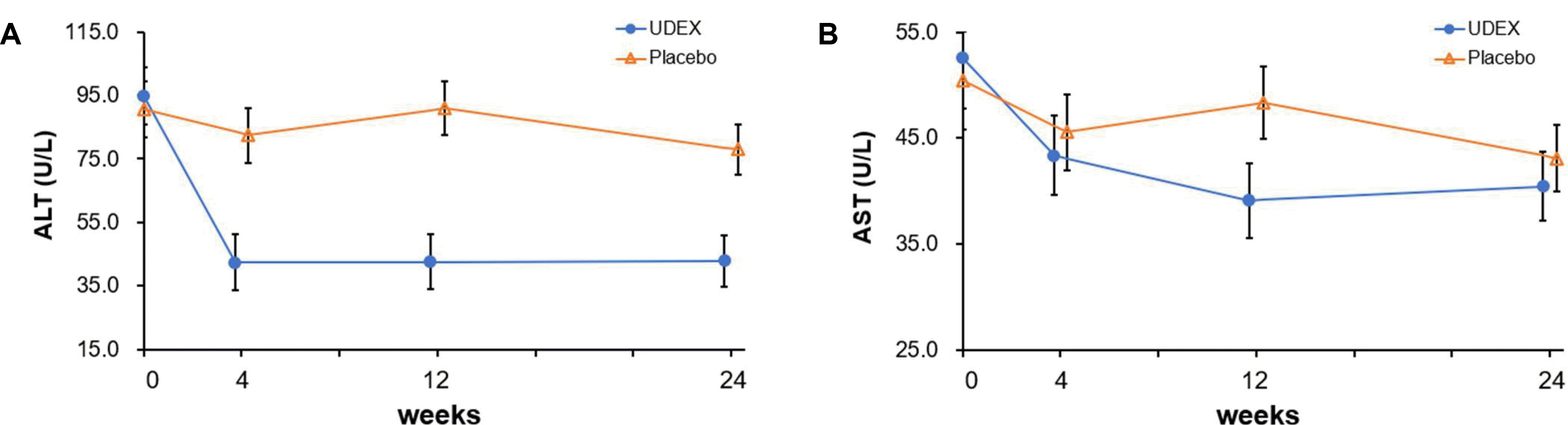
In this double-blind, randomized, placebo-controlled phase 4 trial, the combination drug, including BDD and UDCA, led to ALT normalization in half of the 16 patients (50%) with chronic liver disease related to metabolic syndrome. This proportion was steeply elevated to 73% in those for whom the medication was completed over the 24-week study. Moreover, this change in ALT level during the study period was confirmed after adjusting for the change in BMI.
BDD is a synthesized intermediate derivative of schisandrin C extracted from
Schisandria chinenesis,
8 and has shown ALT reduction in chronic active liver diseases
9 and in protecting against carbon tetrachloride, D-galactosamine, and thioacetamide-induced hepatic damage.
10,11 BDD combined with garlic oil reduced ALT level rapidly and produced significantly higher ALT normalization rates after 4, 8, and 12 weeks than the placebo group among patients with chronic hepatitis, of which NALFD was the dominant cause.
12
UDCA is a natural, hydrophilic bile acid that normally constitutes ~3% of the human biliary pool.
13 Patients with primary biliary cholangitis treated with UDCA showed significant improvements in the serum levels of bilirubin, alkaline phosphatase, AST, and GGT in a multicenter, controlled trial.
14 Another trial reported that high-dose UDCA (28-35 mg/kg) improved the aminotransferase level, serum fibrosis markers, and selected metabolic parameters in non-alcoholic steatohepatitis (NASH). On the other hand, these benefits have not been confirmed in histologic endpoints.
15
Recently, several potential drugs for NASH have been evaluated. Pioglitazone and vitamin E showed improvements in serum aminotransferases, hepatic steatosis, and lobular inflammation in NASH without DM but had little effect on hepatic fibrosis.
16 On the other hand, pioglitazone may provoke weight gain, pedal edema, and precipitation of congestive heart failure in high-risk patients. Concerns about prostate cancer after long-term use of vitamin E have been raised.
Obeticholic acid showed improvement in the histologic findings of NASH, but an abnormality of the lipid metabolism also occurred.
17 A glucacon-like peptide-1 analog, liraglutide, showed improved NASH more than the placebo did, but its effect was concomitant with weight loss; hence, the improvement of NASH may not have originated from the hepatoprotective effect of this drug or from the weight loss.
18
According to a mouse experiment on chemically induced liver injury, BDD administration decreased the hepatic fatty degeneration induced by alcohol.
19 Another animal study reported that BDD had a potent antioxidant effect in the normal and injured liver by inhibiting the signaling caspases and TNFα.
20 In a methionine-choline-deficient model of rodent NASH, the animals treated with UDCA experienced a marked reduction in steatosis, inflammation, and hepatic cell injuries, such as hepatocyte necrosis and ultrastructural changes.
21 Furthermore, in diabetic mice fed a high-fat diet, UDCA improved hepatic insulin resistance and reduced the steatosis and hepatic cholesterol content significantly.
22 These preclinical studies suggested that BDD and UDCA might improve the liver function in NASH, but there is controversy as to whether each drug has a significant effect in clinical settings.
At this point, some researchers are asking whether the combination of conventional hepatotonic agents, such as BDD and UDCA, might induce some improvement in NASH or similar conditions, but there is limited data available.
In 2004, a phase 3 trial showed that more patients with a BDD/UDCA combination reached ALT normalization than with a BDD monotherapy in chronic hepatitis, in which HBV infection was the leading cause.
6 Therefore, this study first showed that a BDD/UDCA combination improved the serum ALT level in chronic hepatitis related to metabolic syndrome with background steatohepatitis. In contrast to previous studies, this study included patients with a hazardous drinking history and controlled viral hepatitis. Recently, there has been a large population with a drinking history but presented similar clinical features to NASH instead of typical alcoholic hepatitis. Furthermore, because more hepatitis B and C patients could be treated by antiviral agents, more patients have lived without viremia but with metabolic syndrome, which often accompanies steatohepatitis. Therefore, the benefits of BDD/UDCA combination in this study could be applied to a broader range of patients.
Despite the improved serum ALT level, the BDD/UDCA combination did not improve the non-invasive liver fibrosis, or liver steatosis marker. This finding corresponded to a previous report that BDD showed rapid normalization of the serum ALT but had no beneficial effect on the histological grade and stage of liver disease after 12 months of medication.
23 Moreover, this study drug did not improve the quality of life markers for chronic liver disease more than the placebo. This suggests that the BDD/UDCA combination might improve the necro-inflammation in steatohepatitis but could not alter the pathophysiology of steatohepatitis related to metabolic syndrome itself.
This study has several limitations. First, it did not include enough subjects according to the planned sample-size calculation to verify the efficacy of the study drug as a primary endpoint. On the other hand, the calculated post hoc power with the current study result was 84%, which is slightly better than the desired power of 80%. Furthermore, ITT and PP analysis showed a significant difference in ALT normalization in this study, suggesting that this preliminary result should be confirmed by a further study that includes more subjects.
Second, five subjects in the intervention group withdrew from the study, which was more frequent than in the placebo group. Among these subjects, three withdrew consent after enrollment, and two could not be contacted. The three subjects who withdrew their consent did not complain of any adverse effects; hence, their withdrawal did not contribute to the safety issue of the study drug. In addition, a significant difference in the primary endpoint in ITT analysis despite this follow-up loss suggests that the study drug has significant efficacy in a conservative interpretation.
Third, the histology findings of the status of steatohepatitis and fibrosis were not checked. Therefore, it is unclear ALT normalization was reflective of steatohepatitis in this setting.
Finally, the previous medications of DM and dyslipidemia at enrollment were allowed; thus, it was impossible to evaluate the effect of the study drug on the serum glucose and cholesterol in this study.
In conclusion, this study suggests that the combination of BDD and UDCA might induce ALT normalization in steatohepatitis related to metabolic syndrome, which physicians often encounter in their clinic. On the other hand, it is unclear if this combination could prevent or reverse the progression of chronic liver disease in hard endpoints, such as liver fibrosis. Therefore, the long-term efficacy and safety of this population for this point need to be evaluated in a more sophisticated study in the future.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download