An 85-year-old man visited the Seoul Paik Hospital because of general weakness and right upper quadrant abdominal pain that began 10 days prior. He was diagnosed with sigmoid colon cancer and underwent a laparoscopic anterior resection 10 years earlier. A physical examination revealed a blood pressure of 132/78 mmHg, a body temperature of 36.6℃, and a heart rate of 90 beats/min. The laboratory data revealed a hemoglobin level of 9.1 g/dL, white blood cell count of 9,470/mm
3, platelet count of 144,000/mm
3, AST of 57 U/L, ALT of 73 U/L, ALP of 250 U/L, albumin of 2.7 g/dL, PT of 15.3 seconds, total bilirubin level of 1.54 mg/dL, CRP of 19.4 mg/dL, and procalcitonin level of 2.69 ng/mL. Abdominal contrast-enhanced CT showed a 17 cm-sized liver abscess with internal septa in the right lobe (
Fig. 1). The patient underwent ultrasound-guided percutaneous catheter drainage (PCD) and empirical antibiotic therapy. On hospital day 2, the patient complained of abdominal pain with increased severity. A physical examination revealed abdominal tenderness and rebound tenderness of the entire abdomen, with a body temperature of 38.5°C. An abdominal CT scan showed leakage of the liver abscess in the peritoneal cavity, pleural space, and pararenal space (
Fig. 2A, B). The PCD tip was located in the perihepatic space, not the abscess (
Fig. 2C, D). Surgical treatment was considered risky because of the patient's age and performance status (European Cooperative Oncology Group scale 4). The PCD was repositioned, and imipenem/cilastatin was maintained as empirical antibiotics. Consecutive drained pus cultures were positive for
Klebsiella pneumoniae (
K. pneumoniae), and the strain was sensitive to imipenem. Follow-up CT showed that the abscess decreased in size from 17 cm to 13 cm (
Fig. 3). The patient’s abdominal pain and tenderness improved as the PCD and intravenous antibiotics were continued. As observed through the blood test results, the levels of CRP and procalcitonin decreased gradually. On hospital day 44, an abdominal CT revealed a significant decrease in abscess size and reduced fluid collection in the peritoneal cavity and pararenal space (
Fig. 4). On hospital day 55, the PCD was removed, and the patient had recovered fully from peritonitis and PLA.
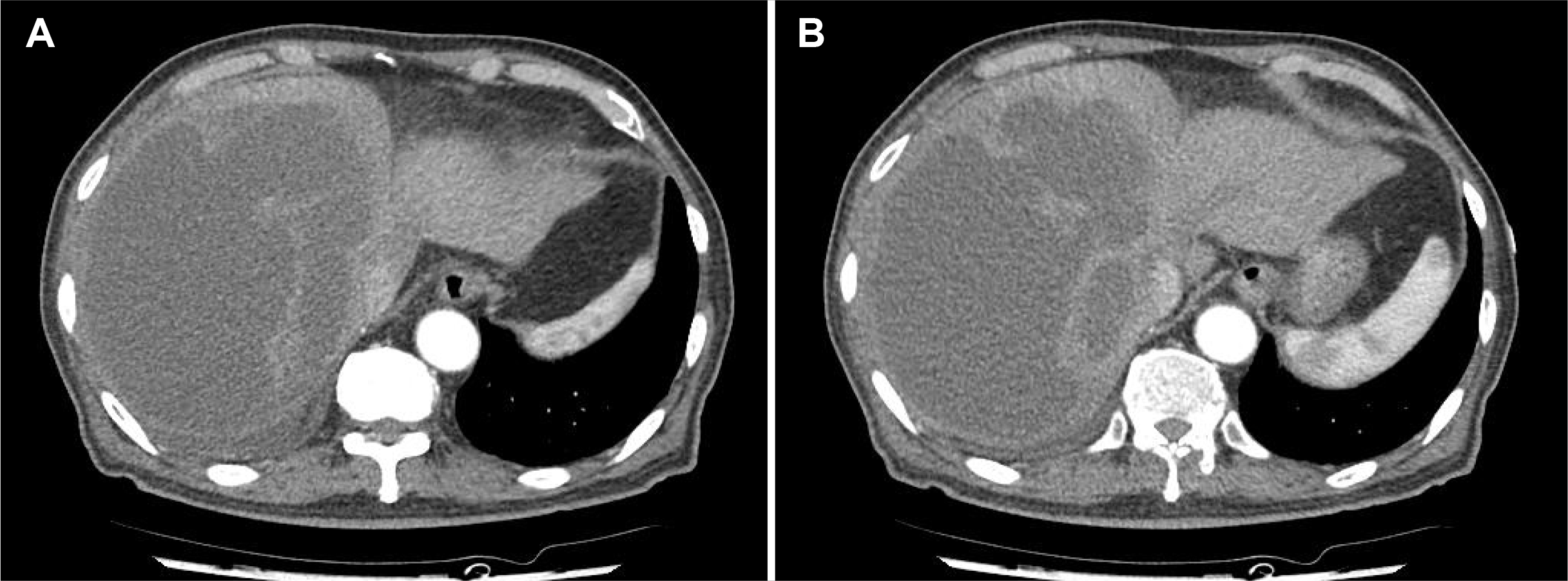 | Fig. 1Computed tomography (CT) scan of the abdomen. (A, B) Initial abdominal CT shows massive, multiple abscesses in the right lobe of the liver. 
|
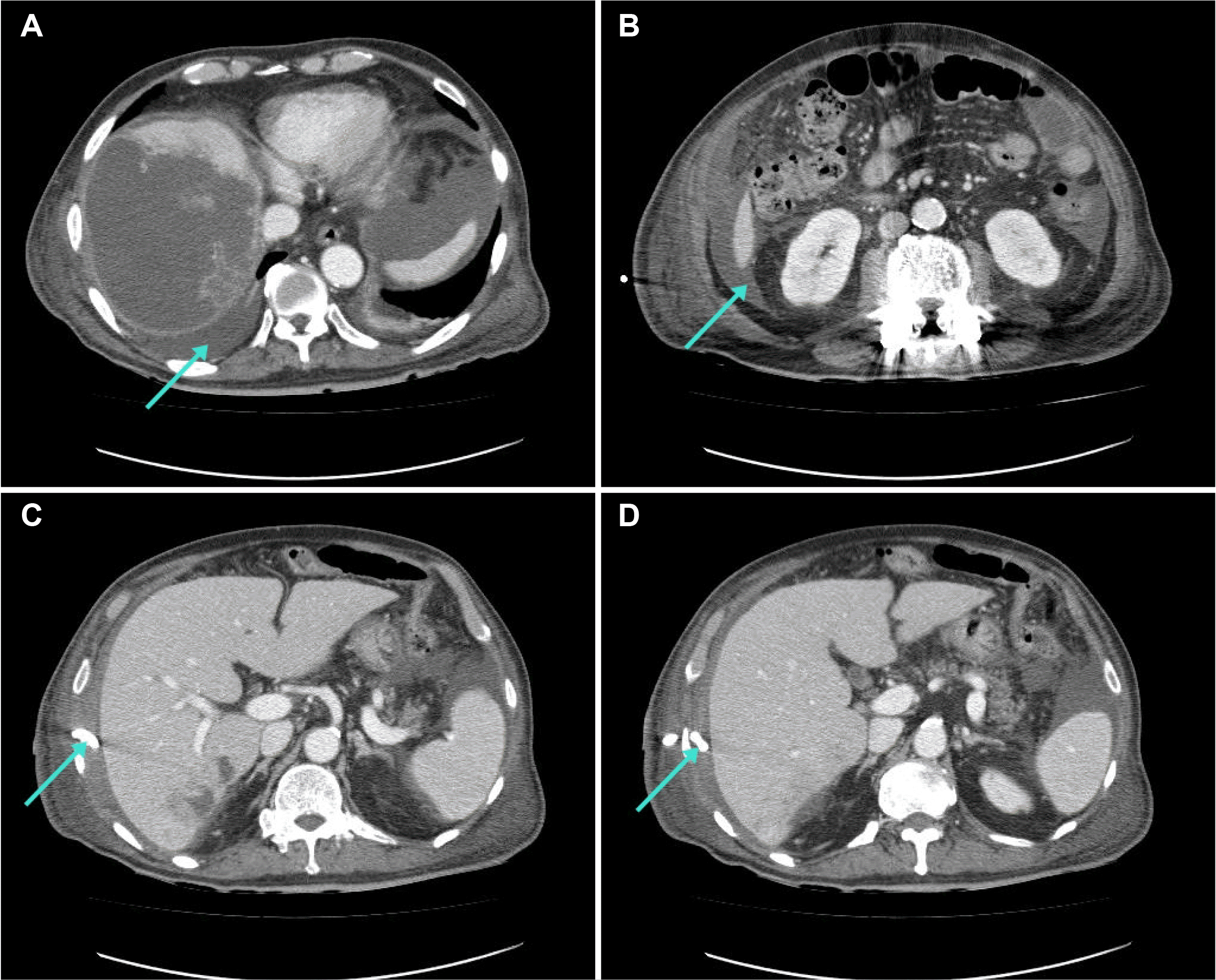 | Fig. 2Computed tomography (CT) scan of the abdomen. (A, B) On hospital day 2, a CT scan revealed rupture of the liver abscess in the peritoneal cavity, pleural space, and pararenal space (arrow). (C, D) The percutaneous catheter drainage tip is located in the perihepatic space (arrow). 
|
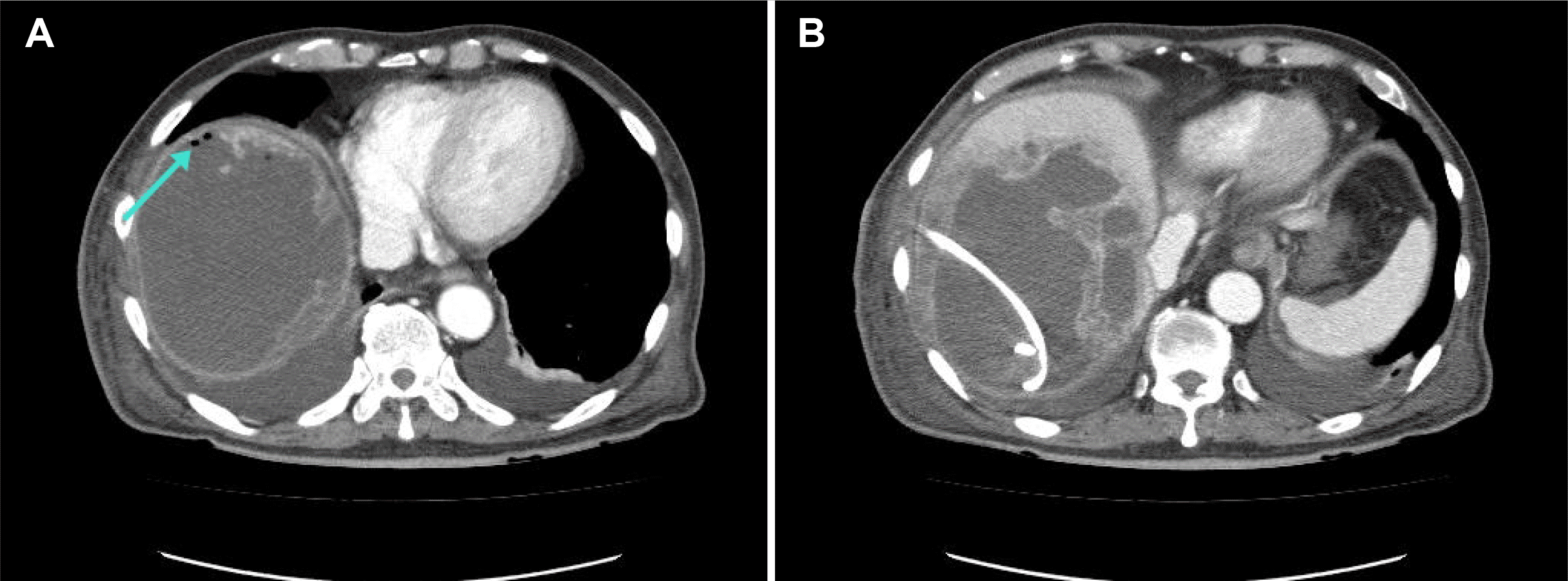 | Fig. 3Computed tomography (CT) scan of the abdomen. (A, B) On hospital day 6, the CT scan showed air bubbles (arrow of A) and a decrease in abscess size. 
|
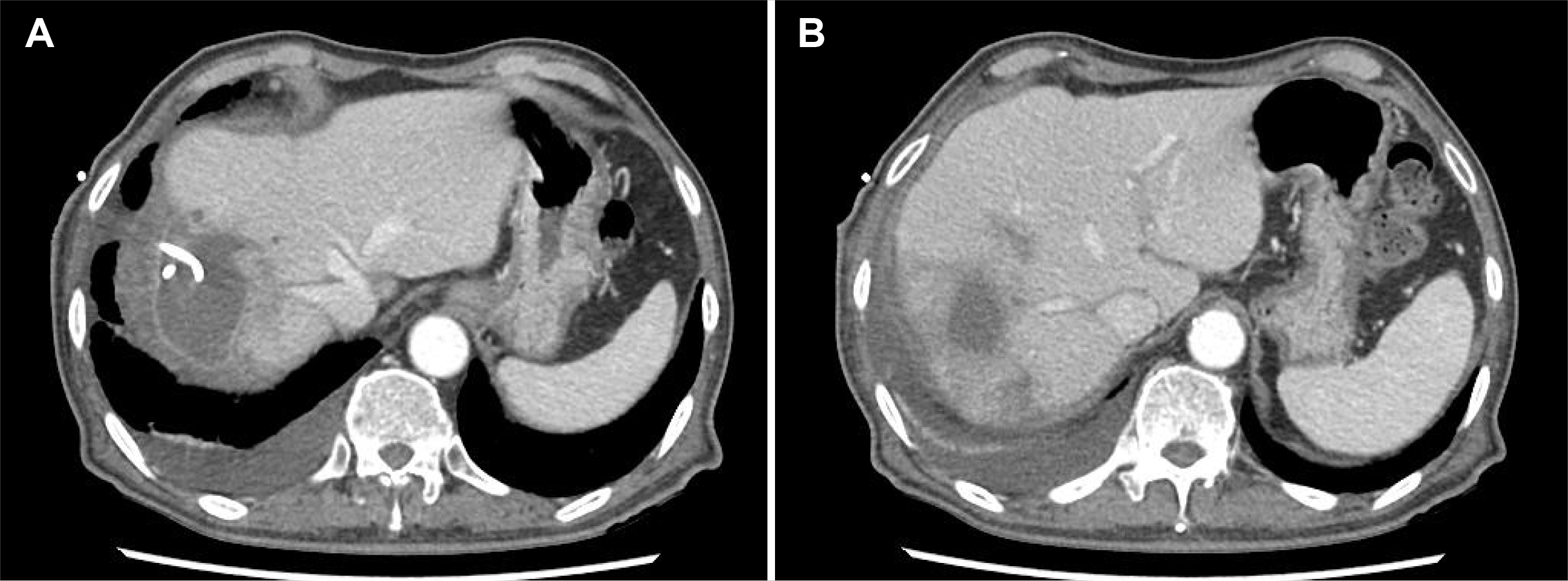 | Fig. 4Computed tomography (CT) scan of the abdomen. (A, B) On hospital 44, the CT scan shows a significant size reduction of the abscess and diminished fluid collection. 
|






 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download