Abstract
Neonatal hypoxia/ischemia (H/I), injures white matter, results in neuronal loss, disturbs myelin formation, and neural network development. Neuroinflammation and oxidative stress have been reported in neonatal hypoxic brain injuries. We investigated whether Paeoniflorin treatment reduced H/I-induced inflammation and oxidative stress and improved white matter integrity in a neonatal rodent model. Seven-day old Sprague–Dawley pups were exposed to H/I. Paeoniflorin (6.25, 12.5, or 25 mg/kg body weight) was administered every day via oral gavage from postpartum day 3 (P3) to P14, and an hour before induction of H/I. Pups were sacrificed 24 h (P8) and 72 h (P10) following H/I. Paeoniflorin reduced the apoptosis of neurons and attenuated cerebral infarct volume. Elevated expression of cleaved caspase-3 and Bad were regulated. Paeoniflorin decreased oxidative stress by lowering levels of malondialdehyde and reactive oxygen species generation and while, and it enhanced glutathione content. Microglial activation and the TLR4/NF-κB signaling were significantly down-regulated. The degree of inflammatory mediators (interleukin 1β and tumor necrosis factor-α) were reduced. Paeoniflorin markedly prevented white matter injury via improving expression of myelin binding protein and increasing O1-positive olidgodendrocyte and O4-positive oligodendrocyte counts. The present investigation demonstrates the potent protective efficiency of paeoniflorin supplementation against H/I-induced brain injury by effectually preventing neuronal loss, microglial activation, and white matter injury via reducing oxidative stress and inflammatory pathways.
Hypoxia/ischemia (H/I) induced brain injury due to lack or insufficient supply of glucose and oxygen to the brain is leading causes of neonatal morbidity and mortality with a higher occurrence rate of 2–6 per 1,000 births [1]. Neonatal H/I results in white matter injury, neuronal cell loss, impaired neuronal function due to failure of neural network subsequently leading to neurological impairments [2,3] as motor and cognitive disabilities, epilepsy, and autonomic dysfunction [4-6]. White matter is found to be highly vulnerable to injury following H/I insult. H/I-induced loss of oligodendroglia, axonal damage, and impaired myelin formation have been reported in the pathogenesis of cerebral palsy and neurobehavioral deficits [7-10].
OPCs of the developing brain are predominantly liable to H/I insults [11,12]. Loss of early OPCs and failure to differentiate to mature OPCs disrupt myelination in the immature brain [12]. Developing brain is extremely susceptible to oxidative stress due to increased rate of oxygen utilization and low levels of endogenous antioxidant defense and high amounts of fatty acids [13,14]. Studies have suggested oxidative stress significantly contributes to the loss of early OPCs [12,15].
Neuroinflammatory responses, characterized by activation of microglial cells, have been observed in neonatal hypoxic brain injuries [16,17]. Accumulating experimental data reveals that neuroinflammatory processes are critically involved in H/I-induced loss of early OPCs in developing brain [18-20]. The microglial cells are involved in the regulation of proliferation of neurons and oligodendrocytes [21,22]. Toll-like receptor 4 (TLR4) is expressed on microglia and mediates neuroinflammatory diseases [23]. Increased expression of TLR4 in microglia following hypoxic insult has also been reported in vitro [21]. Pro-inflammatory cytokines such as interleukin 1 (IL-1), IL-6 and tumor necrosis factor-alpha (TNF-α) are generated by activated microglial cells. These proinflammatory cytokines are regarded as critical players in H/I-induced white matter damage in neonatal brain [24,25]. Excessive production of these cytokines has been reported to disrupt myelin formation, and also contribute to the loss of OPCs [18,26]. Thus, strategies that prevent/or reduce microglial activation and neuroinflammatory process and oxidative stress can prevent the loss of OPCs and aid in restoration of white matter integrity and brain development (Supplementary Fig. 1).
Paeoniflorin, monoterpene glucoside, is one of the primary bioactive compounds of the plant, Paeonia lactiflora. Paeoniflorin is reported to possess anti-cancer, immunomodulatory and neuroprotective effects [27-30]. In this investigation, we assessed the effects of systemic administration of Paeoniflorin in H/I induced neonatal rodents.
Isoflurane and paeoniflorin was obtained from Sigma-Aldrich, St. Louis, MO, USA. Antibodies against cleaved caspase-3, TLR4, TNF-α, Bad, Bcl-2, β-actin and hypoxia-inducible factor-1 alpha (HIF-1α) (Cell Signaling Technology, Beverly, MA, USA). For expression analysis NF-κBp65, IκBα, p-IκBα, myelin binding protein (MBP) (Santa Cruz Biotechnology, Santa Cruz, CA, USA), were utilized. Most other reagents and chemicals were purchased from Sigma-Aldrich, St. Louis, MO, USA indicated otherwise.
Female, pregnant Sprague Dawley rats (n = 25) were obtained from the Institution Animal breeding center and were kept separately in sterile polypropylene rat cages under controlled animal house conditions (temperature 22°C–23°C; relative humidity 55%–60%; 12 h/12 h day-night cycle). The rats were strictly observed for pups to be born and the day of birth was noted as postnatal day 0 (P0). The experimental design and the procedures were endorsed by the Institutional ethical committee, and care and handling of the animals were in strict adherence to the guidelines issued by the National Institutes of Health on the care and use of animals [31].
P3 pups were randomly assigned to study groups (6 groups; n = 24/group). Group I - control; Group II - H/I control; Group III to V - H/I induced and treated with paeoniflorin at 6.25 mg/kg body weight (b.wt), 12.5 mg/kg b.wt and 25 mg/kg b.wt respectively. Group VI pups were not exposed to H/I insult but were treated with 25 mg/kg b.wt paeoniflorin.
H/I was induced in P7 pups as described earlier by Rice et al. [32]. In brief, the P7 pups for induction were exposed to 3% isoflurane anaesthesia and for maintenance 1.5% isoflurane was used. The left common carotid artery was separated carefully and ligated twice with 6–0 surgical silk to block the flow of blood. The pups were allowed to recover for 2 h and were subjected to oxygen (8%) in nitrogen gas mixture in a humidified chamber. The rats were maintained at 37°C by heating blanket that was thermostatically controlled. After hypoxia induction, the pups were allowed to breathe normal air for 5 to 10 min, following which the pups were returned to their dams.
P3 pups were administered paeoniflorin (6.25, 12.5, 25 mg/kg b.wt), orally via gavage from day P3 and extended till P14. Paeoniflorin was administered an hour before H/I induction to the pups on P7. The dosage used was selected based on the results obtained by screening various doses of paeoniflorin (1 mg/kg to 100 mg/kg b.wt) at our laboratory. We received no significant changes in the vital parameters and were found not to exert any cytotoxic effects (data not included).
The rat pups were sacrificed on P8 (n = 6/group), P10 (n = 6/group) and on P14 (n = 6/group) under isoflurane anaesthesia by transcardial perfusion of saline-heparin tailed by ice-cold paraformaldehyde solution in 0.1 M PBS. Brains were excised immediately and used for analysis.
Neurobehavioral deficits were assessed in H/I induced rats on P8, P14 and P21. The extent of the neurological deficit was graded based on parameters as - symmetry of movements, symmetry of the forelimbs, spontaneous activity, climbing, touch response, and vibrissae touch response. The behaviour of animals was graded on a scale between 0–4. The animals were scored as: 0 - no deficit (normal); 1 - mild deficit; 2 - moderate deficit; 3 - severe deficit; and 4 - very severe deficit [33,34].
Tissue viability and infarct size was measured following 24 h after H/I on P8 and P14 day. Brain tissue sections of 5 µm thickness (n = 6/group; n = 4/animal) were cut at the level of interaural distance 5.40 mm and bregma 3.60 mm. The sections were embedded in paraffin were incubated with cresyl violet stain. Tissue sections were visualised (Carl Zeiss Stemi 2000-C stereomicroscope; Zeiss, Oberkochen, Germany) and the infarcted location was measured with NIH ImageJ software (Version1.42; National Institutes of Health, Bethesda, MD, USA). The infarcted region was unstained, while normal areas appeared stained with cresyl violet. The extent of infarction was determined as follows infarct area (%) = ([C – I] / C) × 100; C - mean of the contralateral area and I - mean value of the ipsilateral area.
Staining with terminal transferase-mediated dUTP nickend-labeling (TUNEL) was done to evaluate neuronal apoptosis following H/I. Sections of tissues measuring about 5 µm thickness (n = 6/group) were treated as per the instructions specified (DeadEnd fluorometric TUNEL system kit; Promega, Madision, WI, USA) and were analysed for apoptotic cells by NIS-Elements BR imaging processing and analysis software (Nikon Corporation, Tokyo, Japan).
Immunohistochemical examination was done to determine the effects of H/I on myelination in H/I induced pups. The assessments were done on P8 and P14. The excised brains that were post-fixed and equilibrated in 30% sucrose in 0.1 M PBS were used for analysis. Coronal sections (5 µm thickness) sliced at interaural distance 5.40 mm and bregma −3.60 mm level using microtome (Leica 1325; Leica Biosystems, Nussloch, Germany). The sections were cleaned with PBS and then treated with 1% H2O2 in PBS and 0.25% Triton X-100 in PBS (blocking solution) to exclude any activity of endogenous peroxidase. The sections were cleaned with PBS and incubated with specific primary antibodies against MBP, pre-myelinating oligodendrocytes (anti-O4; clone 81; Millipore, Billerica, MA, USA), immature oligodendrocytes (anti-O1; clone 59; Millipore) and incubated at 4°C overnight. Following incubation with primary antibodies, the sections were cleaned thrice with PBS and treated with horse-radish peroxidase tagged secondary antibodies for 1 h, treated with diaminobenzidine (DAB) and hematoxylin counterstained. The tissue sections were then dehydrated in ethanol and treated with xylene. The immunolabeled tissue sections were visualised and analysed using a light microscope (Olympus BX 50; Olympus, Tokyo, Japan).
Microglial activation was determined by assessing the expression of ionized calcium binding adaptor molecule 1 (Iba-1) as previously mentioned by Arteaga et al. [38]. The brain tissue sections (60 µm) thickness were blocked for peroxidase activity and then treated with anti-Iba-1 primary antibody at 4°C (Abcam, Cambridge, UK) overnight. The segments were cleaned twice with PBS and incubated further with secondary antibody conjugated with Alexa 488 (Abcam) and then counterstained with DAPI. The tissue sections were examined using a Confocal Microscope (Olympus Fluoview FV500; Olympus).
Excised brain tissues (n = 6/group) were blended using cell lysis buffer from Cell Signaling Technology, USA (20 mM Tris-HCl [pH 7.5], 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 150 mM NaCl, 1 mM Na3VO4, 1 µg/ml leupeptin, 1 mM Na2EDTA, 1 mM beta-glycerophosphate). The whole cell extracts obtained were subjected to centrifugation (3,000 rpm; 15 min) at 4°C. Supernatant collected was used for analysis. Cytosol and nuclear fractions were separated from the whole cell homogenate using ReadyPrep Protein Extraction Kit (Cytoplasmic/Nuclear) from Bio-Rad (Hercules, CA, USA). Total protein content of the whole cell extracts and in cytosol and nuclear fractions were quantified using kits from Thermo Fischer Scientific (Waltham, MA, USA). For Western blot analysis 60–80 µg amount of protein sample (n = 6/group) were loaded on SDS-PAGE (10%–15%) gels and were electrophoresed. The separated protein bands were blot transferred on to polyvinylidinene fluoride (PVDF; Thermo Fischer Scientific) and the membranes were blocked in 5% non-fat dry milk in 1x TBST to exclude any endogenous peroxidase activity. After washing with TBST, the PVDF membranes were incubated nightlong at 4°C with the primary antibodies. The membranes were cleaned using TBST and treated with secondary antibody (Horse radish peroxidase-conjugated; 1:2,000; Santa Cruz Biotechnology) at room temperature for 60 min. After incubating with secondary antibodies, the membranes were cleaned again with TBST and the bands were observed using enhanced chemiluminescence system (Millipore). The positive bands obtained were scanned and analysed using Image J software (Supersignal, Pierce, IL, USA).
Concentrations of pro-inflammatory mediators - IL-1β and TNF-α in the brain tissues post H/I insult were determined by ELISA. The fore brain tissues were homogenised in 0.1 M ice-cold Phosphate buffer and centrifuged. The supernatant was used for analysis of total protein content and for TNF-α and IL-1β levels using kits from RD systems (Minneapolis, MN, USA).
Brain tissue samples (n = 6) were homogenized from each treatment group with 1:10 (w/v) PBS on ice and centrifuged (3,000 rpm; 15 min, 4°C). Accumulated supernatant was used for determination of ROS generation, lipid peroxidation and glutathione (GSH) levels. Total quantity of protein in supernatant was found using Bradford protein assay kit (Bio-Rad). Levels of ROS in the brain tissues on P14 was detected using in vitro ROS/RNS assay kit (OxiSelect) from Cell Bio-Labs Inc. Dichlorodihydrofluorescin DiOxyQ (DCFH-DiOxyQ), a fluorogenic probe specific to ROS/RNS was employed. The fluorescence intensity is measured using Synergy 2 Multi-function Microplate Reader.
The P8, P14 and P21 rats that were exposed to hypoxic insult on P7 were assessed for behavioural responses and neuromuscular coordination. The H/I induced rats exhibited difficulties to walk and to move around indicating neurological deficits (Fig. 1). The flexibility of the left limbs was found to be reduced and the rats struggled to respond to the test stimulus. The neurological deficits were observed to be substantively (p < 0.05) reduced in animals that were treated with paeoniflorin at 6.25, 12.5 and 50 mg doses. Furthermore, on day P21 rats treated with paeoniflorin noticeably (p < 0.05) exhibited development in the neuro-behavioural responses and in muscular coordination vs. paeoniflorin treated rats at P8 and P14. Further, the behaviour of the group 6 animals that were administered with paeoniflorin at 25 mg/kg dosage and not subjected to hypoxic insult did not exhibit any neurological deficits. The behaviour of the rats was similar to that of normal control.
Infarct area following H/I was determined by cresyl violet staining. Infarcted regions appeared as unstained regions vs. normal regions stained with cresyl violet. The ischemic areas were observed predominantly in the striatum and in the fronto-parietal cortex regions (Fig. 2A, B). Pre- treatment with paeoniflorin at 6.25, 12.5 and 25 mg doses resulted in a substantially (p < 0.05) reduced infarct volume following H/I (Fig. 2A, B). Nevertheless, supplementation of 25 mg dose more expressively (p < 0.05) decreased cerebral infarct size in comparison to lower doses. In P8 rats, the infarct volume decreased from 68.15% to 15.72% and in P10 rats the infarct volume reduced to 10.08% and in P14 rats the infarct volume reduced to 5.79% with paeoniflorin treatment at 25 mg. Further, group 6 rats that were treated with paeoniflorin at 25 mg alone did not exhibit any neuronal injury. Also, interestingly, irrespective of the dosage given, the infarct size was significantly reduced in rats.
Neuroprotective effects of paeoniflorin pre-treatment was further evaluated by TUNEL assay. The data obtained illustrated that administration of paeoniflorin produced a significant (p < 0.05) decline in H/I-induced neuronal cell death (Fig. 3A, B). The apoptotic cell counts decreased from 149 cells/mm2 in H/I control to 52 cells/mm2 on paeoniflorin treatment at 25 mg/kg dose in P8 rats, 19 cells/mm2 in P10 rats and 12 cells/mm2 in P14 rats.
To evaluate further the neuroprotective effects of paeoniflorin treatment prior and post H/I induction, the expression of the proteins associated with cell apoptosis were evaluated by Western blotting in P8 rats exposed to H/I on P7 (Fig. 4A, B). Up-regulated expression of Bad and Cleaved caspase-3 were noticed in the H/I control group vs. normal control. The expression of Bcl-2, the anti-apoptotic protein was detected to be reduced in P8 rats. The enhanced expression of Bad and cleaved caspase-3 observed could have contributed to increased apoptotic cell counts as noticed in TUNEL assay. Interestingly, while elevated expression of Bcl-2 was noticed on paeoniflorin treatment, decreased expression of Bad and cleaved caspase-3 vs. H/I control were observed (Fig. 4A, B). Bcl-2 expression increased to 66.3%, 78.14%, and 85% on treatment with 6.25, 12.5, and 25 mg paeoniflorin respectively. Cleaved caspase-3 expression decreased to 18.10% in H/I-induced rats treated with 25 mg paeoniflorin. Modulation of apoptotic protein expression by paeoniflorin could have aided in reduced neuroapoptotic cell counts.
H/I-induced oxidative stress is well documented in H/I-induced brain injury [35]. ROS and lipid peroxidation levels in brain tissues were measured in P8 pups at 24 h following induction of H/I and in P10 rats at 72 h post H/I insult. A multi-fold increase in ROS and MDA levels following H/I was observed (Fig. 5A, B). ROS generation was found to increase to 375.08% in H/I control on P8 and to 407.08% in P10 rats vs. normal control group. MDA content was observed to raise to 21.30 nM/mg protein at 24 h and to 29.63 nM/mg protein at 72 h following H/I insult vs. 1.17 nM/mg protein in normal control. Interestingly, treatment with paeoniflorin at all the dosages tested decreased ROS and MDA levels. ROS generation decreased to 118.09% (P8) and 125.1% (P10) with 25 mg treatment vs. 375.08% in H/I control. In line with ROS, MDA levels were also reduced on paeoniflorin treatment when compared to H/I control. MDA levels reduced to 8.12, 5.07, 2.1 nM/mg protein in P8 rats on treatment with 6.25 mg, 12.5 mg, and 25 paeoniflorin respectively.
GSH levels in H/I control rats were found to be reduced (p < 0.05) in H/I-induced P8 rat pups (Fig. 5C). Twenty four hours post H/I induction, GSH content decreased to 13.76 nM/mg protein in H/I control vs. 38.19 nM/mg protein in normal control group. Supplementation of paeoniflorin to pups from P3 resulted in a markedly raised GSH content in the brain tissues. The levels increased to 15.37 nM/mg protein, 29.28 nM/mg protein and 40.33 nM/mg protein on treatment with 6.25 mg, 12.5 mg, and 25 paeoniflorin respectively. P10 rats exposed to H/I insult and treated with paeoniflorin at 25 mg also exhibited a significant increase in GSH levels. Further, group 6 rats that were treated with 25 mg paeoniflorin alone presented GSH levels of 39.78 nM/mg protein (P8 rats) and 42.74 nM/mg protein (P10 rats). The levels were comparable to that of normal control.
Activation of microglia were assessed by determining the expression of the macrophage marker Iba-1. H/I-induction resulted in a significant (p < 0.05) rise in Iba-1-postive cells (Fig. 6A). Activated microglial cells were observed in the dentate gyrus and in the hippocampal CA 1 areas in the H/I control animals vs. normal controls. In contrast, Iba-1 expression levels were noticed to be remarkably decreased in P8 and in P10 pups treated with paeoniflorin at all the 3 tested doses compared to H/I control.
TLR4 levels were seen to increase multi-fold post H/I to 205.10% in P8 and to 280.26% in P10. HIF-1α levels were elevated to 190% in P8 and to 289.1% at P10. The up-regulated expression of TLR4 and HIF-1α observed following 24 h and 72 h of H/I induction were down-regulated significantly (p < 0.05) in rats supplemented with paeoniflorin (Fig. 6B). TLR4 and HIF-1α expression reduced to 129% and 115.2% respectively in P8 rats administrated with 25 mg Paeoniflorin. These observations suggest that paeoniflorin effectively reduced microglial activation.
NF-κB signaling is known to be activated under hypoxic conditions [36]. H/I induction in P7 rats caused a markedly enhanced (p < 0.05) nuclear NF-κB (p65) expression with substantially (p < 0.05) lower cytosolic levels of NF-κB (p65) (Fig. 7). The observations indicate activation of NF-κB. Furthermore up-regulated expression of TNF-α, and levels of phosphorylation of the regulatory kinase-IκBα were noted both at 24 h and 72 h post H/I. However, we noticed a marked increase in nuclear NF-κB (p65) expression at P10 when compared at 24 h. Paeoniflorin supplementation at all the 3 doses resulted in a significant suppression (p < 0.05) of NF-κB p65 (nuclear fraction) expression vs. H/I control. 25 mg dose of paeoniflorin significantly (p < 0.05) reduced the expression of NF-κB p65 in the nuclear fraction from 191.21% to 119.23% at P8 and from 235.10% to 139.27% at P10. Paeoniflorin at the tested doses of 6.25 mg/kg, 12.5 mg/kg and 25 mg/kg down-regulated p-IκBα expression vs. H/I control group at both P8 and P10. These observations suggest the inhibit activation of NF-κB signaling by paeoniflorin.
Brain levels of proinflammatory cytokines - IL-1β and TNF-α at 24 h after H/I were observed to be significantly raised (p < 0.05) vs. normal control (Fig. 8). Paeoniflorin pre-treatment from P3 resulted in a significant drop in the concentration of IL-1β and TNF-α.
White matter integrity post H/I insult was detected by calculating the density of MBP positive cells by immunohistochemical analysis. A significant reduction in MBP positive cell counts were observed at 24 h following H/I. MBP positive counts was noticed to be reduced by 29.12% at 24 h and 18.08% at 72 h post H/I in the in the corpus callosum of H/I control rats in comparison to normal control rats (Fig. 9). Prior treatment with paeoniflorin from P3 significantly (p < 0.05) improved MBP counts from 29.12% to 37.24%, 49.33% and 68.79% with 6.25 mg, 12.5 mg and 25 mg doses respectively at P8. Paeoniflorin supplementation at 25 mg improved MBP counts to 71.23% at P10. However, paeoniflorin alone administered rats exhibited no significant difference in MBP-positive cell counts on P8 and P10.
OPCs proliferation and maturation are critical for myelination. We noticed a significant decrease in the O4-positive oligodendrocytes and O1-positive oligodendrocytes at 24 h and 72 h following H/I insult in P8 and in P10 rat pups. Paeoniflorin treatment was found to effectively reduce the loss of OPCs (Fig. 9). In P8 pups, paeoniflorin at 25 mg significantly (p < 0.05) improved the counts of O4-positive oligodendrocytes to 89.67% from 16.14% in H/I control and O1-positive counts to 93.10% vs. 18.34% in H/I control group. The results reveal that paeoniflorin effectively prevented neuronal loss and improved myelination and white matter integrity.
Perinatal H/I-induced brain injury is the major cause of death and long-term neurological impairments [37]. The pathogenesis of H/I-induced brain injury involves several mechanisms- including, neuroinflammation, oxidative stress and apoptosis [13,18,38]. Lack of effective treatment strategies makes the identification of novel compounds inevitable. The present research was aimed to study the effects of systemic administration of paeoniflorin in animal model of neonatal H/I brain injury.
Paeoniflorin administered from P3 was found to effectively improve neurobehavioral scores and reduce neuronal apoptosis and infarct volume. Immunoblotting analysis revealed that paeoniflorin significantly down-regulated expression of cleaved caspase-3, chief marker of apoptotic cell death and pro-apoptotic protein, Bad. Interestingly paeoniflorin enhanced expression of anti-apoptotic protein, Bcl-2. The balance between anti-apoptotic and pro-apoptotic proteins is crucial in regulating cell existence [39]. The up-regulated Bcl-2 expression by paeoniflorin was noticed to be in line with decreased TUNEL positive cell counts, reflecting the anti-apoptotic effects of paeoniflorin.
Oxidative stress is well documented as a major contributor of H/I-induced brain injury [38]. Raised levels of free radicals including ROS are seen during the 2nd phase of H/I brain injury that occurs between 6–48 h of H/I insult. These elevated ROS levels extent for days post H/I insult contributing to further complications as chronic neuroinflammation, decreased neurogenesis and cell death [35,38]. In line with the previous reports, increased ROS production and MDA levels were observed in our study reflecting oxidative stress following H/I at 24 h and 72 h. Supressed GSH levels suggest utilisation of antioxidant defences in responses to stress. Paeoniflorin supplementation prior to H/I insult and post insult was found to remarkably reduce ROS production and MDA content and as well increased GSH levels. The observations suggest the anti-oxidant efficacy of paeoniflorin.
Neuroinflammatory processes are pivotal players in the loss of OPCs, loss of white matter integrity and subsequently causing brain injury [40,41]. Microglial cells are primary immune cells in neuroinflammatory responses and are reported to be critically involved in hypoxia-induced neuronal loss [17,42,43]. The Toll-like receptors (TLRs) are involved in innate defence responses and TLR4 expressed on microglia are reported in several neuroinflammatory diseases [16,23]. TLR4 expression was up-regulated following ethanol induced activation of microglia [44]. In our study we noticed marked activation of microglia at 24 h and at 72 h following H/I insult as evidenced by significantly raised levels of the microglial/macrophage marker, Iba-1. In line with expression levels of Iba-1, TLR4 expression was noticed to be up-regulated.
It is known under that the expression of HIF-1α is strongly upregulated under hypoxia [45,46]. HIF-1α is found to regulate TLR4 expression in microglia and macrophages under hypoxia [21,47]. Here again, we noticed up-regulated expression of HIF-1α following hypoxic insult. The elevated TLR4 expression may be due to enhanced HIF-1α.
Interestingly, we noted NF-κB signaling was significantly up-regulated following H/I induction. The results of our study revealed marked nuclear translocation of NF-κB (p65) post H/I. Murugan et al. [36] have reported that hypoxia causes degradation of p-IκB and induces activation of NF-κB signaling by promoting translocation of NF-κB (p65) to the nucleus. NF-κB signaling is one the major pathways involved in neuroinflammatory responses leading to expression of pro-inflammatory mediators including cytokines [48,49]. NF-κB activation is found to be critically involved in microglial TLR4 signaling. TLR4 has been reported to promote the production of inflammatory mediators through NF-κB signaling [47]. Increased levels of inflammatory cytokines and enhanced iNOS levels have been reported following NF-κB activation in microglia following hypoxic insult [50]. As demonstrated in earlier studies [20,47] significantly elevated levels of TNF-α and IL-1β in the brain tissues of pups exposed to H/I insult were noticed. The increased levels reflected activation of NF-κB/TLR4 signaling.
It has been suggested that microglial activation and TLR4/NF-κB-mediated release of inflammatory mediators induce disruption of white matter integrity and subsequently leading to white matter injury [44]. Pro-inflammatory cytokines were found to cause loss of immature OPCs and affect myelination post H/I insult [19,26]. Disruption of myelination occurs due to loss of premyelinating oligodendrocytes and loss of maturation of OPCs [51,52]. OPCs of the immature brain are reported to be specifically vulnerable to H/I insults [11,12]. Low innate antioxidant enzymes in part have reported to contribute to cell death [12,15]. White matter injury is known as a major cause of chronic neurological deficits in H/I survivors [7]. The progenitor cells are pivotal for the replacing the degenerating premyelinated oligodendrocytes and as well in the formation of new myelin. In the present study we noticed significantly reduced MBP expression following H/I insult. Reduced MBP levels are regarded as a marker for white matter injury [53,54]. Similar to the observations of the previous reports, in the present study, marked losses of O4- and O1-positive oligodendrocytes were noticed in P8 and in P10 rat pups that were subjected to H/I insult on P7 [11,18,20].
Paeoniflorin supplementation to the pups prior H/I induction significantly reduced microglial activation as evidenced by decreased Iba1 levels and reduced TLR4 expression. Paeoniflorin down-regulated NF-κB activation in a dose-dependent manner, with 25 mg dose exhibiting higher effects. TNF-α and IL-1β levels were also reduced in the brain tissues suggesting suppression of inflammatory responses. Also, paeoniflorin significantly prevented the loss of O4- and O1-positive oligodendrocytes and improved MBP cell counts in comparison to H/I control group. These observations indicate paeoniflorin prevented white matter injury and improved myelination. Restoration of white matter integrity leads to functional recovery [9]. Thus, paeoniflorin could also aid in neurofunctional improvements post H/I insult. Paeoniflorin significantly reduced oxidative stress and caused down-regulation of HIF-1α. Paeoniflorin-mediated reduction in HIF-1α expression could have partly caused downregulation of TLR4 and thus leading to inhibition of TRL4/NF-κB signaling. Down-regulated NF-κB signaling and inflammatory cytokines by paeoniflorin could have aided in prevention of neuronal loss and white matter injury. The results demonstrate the neuroprotective efficiency of paeoniflorin.
Paeoniflorin pre-treatment significantly attenuated oxidative stress and inhibited up-regulation of TLR4/NF-κB mediated inflammatory responses. Paeoniflorin markedly reduced neuronal loss and white matter injury by improving MBP-positive counts and O4- and O1-positive oligodendrocytes. Our results suggest that paeoniflorin as a potent candidate in therapy of H/I induced neurological impairments.
Supplementary data including one figure can be found with this article online at https://doi.org/10.4196/kjpp.2021.25.2.97.
Notes
REFERENCES
1. de Haan M, Wyatt JS, Roth S, Vargha-Khadem F, Gadian D, Mishkin M. 2006; Brain and cognitive-behavioural development after asphyxia at term birth. Dev Sci. 9:350–358. DOI: 10.1111/j.1467-7687.2006.00499.x. PMID: 16764608.


2. Hamrick SE, Ferriero DM. 2003; The injury response in the term newborn brain: can we neuroprotect? Curr Opin Neurol. 16:147–154. DOI: 10.1097/01.wco.0000063775.81810.79. PMID: 12644741.


3. Edwards AD, Brocklehurst P, Gunn AJ, Halliday H, Juszczak E, Levene M, Strohm B, Thoresen M, Whitelaw A, Azzopardi D. 2010; Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ. 340:c363. DOI: 10.1136/bmj.c363. PMID: 20144981. PMCID: PMC2819259.

4. Anderson P, Doyle LW. 2003; Neurobehavioral outcomes of school-age children born extremely low birth weight or very preterm in the 1990s. JAMA. 289:3264–3272. DOI: 10.1001/jama.289.24.3264. PMID: 12824207.


5. Hack M, Youngstrom EA, Cartar L, Schluchter M, Taylor HG, Flannery D, Klein N, Borawski E. 2004; Behavioral outcomes and evidence of psychopathology among very low birth weight infants at age 20 years. Pediatrics. 114:932–940. DOI: 10.1542/peds.2003-1017-L. PMID: 15466087.


6. Damodaran T, Hassan Z, Navaratnam V, Muzaimi M, Ng G, Müller CP, Liao P, Dringenberg HC. 2014; Time course of motor and cognitive functions after chronic cerebral ischemia in rats. Behav Brain Res. 275:252–258. DOI: 10.1016/j.bbr.2014.09.014. PMID: 25239606.


7. Back SA. 2014; Cerebral white and gray matter injury in newborns: new insights into pathophysiology and management. Clin Perinatol. 41:1–24. DOI: 10.1016/j.clp.2013.11.001. PMID: 24524444. PMCID: PMC3947650.


8. Huria T, Beeraka NM, Al-Ghamdi B, Fern R. 2015; Premyelinated central axons express neurotoxic NMDA receptors: relevance to early developing white-matter injury. J Cereb Blood Flow Metab. 35:543–553. DOI: 10.1038/jcbfm.2014.227. PMID: 25515212. PMCID: PMC4420873.


9. Murray AL, Thompson DK, Pascoe L, Leemans A, Inder TE, Doyle LW, Anderson JFI, Anderson PJ. 2016; White matter abnormalities and impaired attention abilities in children born very preterm. Neuroimage. 124(Pt A):75–84. DOI: 10.1016/j.neuroimage.2015.08.044. PMID: 26318524. PMCID: PMC4791057.


10. Song FE, Huang JL, Lin SH, Wang S, Ma GF, Tong XP. 2017; Roles of NG2-glia in ischemic stroke. CNS Neurosci Ther. 23:547–553. DOI: 10.1111/cns.12690. PMID: 28317272. PMCID: PMC6492766.



11. Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC. 2001; Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci. 21:1302–1312. DOI: 10.1523/JNEUROSCI.21-04-01302.2001. PMID: 11160401. PMCID: PMC6762224.



12. Back SA, Riddle A, McClure MM. 2007; Maturation-dependent vulnerability of perinatal white matter in premature birth. Stroke. 38(2 Suppl):724–730. DOI: 10.1161/01.STR.0000254729.27386.05. PMID: 17261726.


13. McQuillen PS, Ferriero DM. 2004; Selective vulnerability in the developing central nervous system. Pediatr Neurol. 30:227–235. DOI: 10.1016/j.pediatrneurol.2003.10.001. PMID: 15087099.


14. McLean C, Ferriero D. 2004; Mechanisms of hypoxic-ischemic injury in the term infant. Semin Perinatol. 28:425–432. DOI: 10.1053/j.semperi.2004.10.005. PMID: 15693399.


15. Thorburne SK, Juurlink BH. 1996; Low glutathione and high iron govern the susceptibility of oligodendroglial precursors to oxidative stress. J Neurochem. 67:1014–1022. DOI: 10.1046/j.1471-4159.1996.67031014.x. PMID: 8752107.


16. Kadhim H, Tabarki B, De Prez C, Rona AM, Sébire G. 2002; Interleukin-2 in the pathogenesis of perinatal white matter damage. Neurology. 58:1125–1128. DOI: 10.1212/WNL.58.7.1125. PMID: 11940709.


17. Kaur C, Ling EA. 2009; Periventricular white matter damage in the hypoxic neonatal brain: role of microglial cells. Prog Neurobiol. 87:264–280. DOI: 10.1016/j.pneurobio.2009.01.003. PMID: 19428957.


18. Cai Z, Lin S, Fan LW, Pang Y, Rhodes PG. 2006; Minocycline alleviates hypoxic-ischemic injury to developing oligodendrocytes in the neonatal rat brain. Neuroscience. 137:425–435. DOI: 10.1016/j.neuroscience.2005.09.023. PMID: 16289838.


19. Fan LW, Lin S, Pang Y, Rhodes PG, Cai Z. 2006; Minocycline attenuates hypoxia-ischemia-induced neurological dysfunction and brain injury in the juvenile rat. Eur J Neurosci. 24:341–350. DOI: 10.1111/j.1460-9568.2006.04918.x. PMID: 16836639.


20. Carty ML, Wixey JA, Colditz PB, Buller KM. 2008; Post-insult minocycline treatment attenuates hypoxia-ischemia-induced neuroinflammation and white matter injury in the neonatal rat: a comparison of two different dose regimens. Int J Dev Neurosci. 26:477–485. DOI: 10.1016/j.ijdevneu.2008.02.005. PMID: 18387771.


21. Ock J, Jeong J, Choi WS, Lee WH, Kim SH, Kim IK, Suk K. 2007; Regulation of Toll-like receptor 4 expression and its signaling by hypoxia in cultured microglia. J Neurosci Res. 85:1989–1995. DOI: 10.1002/jnr.21322. PMID: 17461416.


22. Kim SY, Choi YJ, Joung SM, Lee BH, Jung YS, Lee JY. 2010; Hypoxic stress up-regulates the expression of Toll-like receptor 4 in macrophages via hypoxia-inducible factor. Immunology. 129:516–524. DOI: 10.1111/j.1365-2567.2009.03203.x. PMID: 20002786. PMCID: PMC2842498.



23. Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, Vartanian T. 2003; Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci U S A. 100:8514–8519. DOI: 10.1073/pnas.1432609100. PMID: 12824464. PMCID: PMC166260.



24. Kadhim H, Tabarki B, Verellen G, De Prez C, Rona AM, Sébire G. 2001; Inflammatory cytokines in the pathogenesis of periventricular leukomalacia. Neurology. 56:1278–1284. DOI: 10.1212/WNL.56.10.1278. PMID: 11376173.


25. Silveira RC, Procianoy RS. 2003; Interleukin-6 and tumor necrosis factor-alpha levels in plasma and cerebrospinal fluid of term newborn infants with hypoxic-ischemic encephalopathy. J Pediatr. 143:625–629. DOI: 10.1067/S0022-3476(03)00531-6. PMID: 14615734.

26. Cai Z, Lin S, Pang Y, Rhodes PG. 2004; Brain injury induced by intracerebral injection of interleukin-1beta and tumor necrosis factor-alpha in the neonatal rat. Pediatr Res. 56:377–384. DOI: 10.1203/01.PDR.0000134249.92944.14. PMID: 15201401.


27. Liu DF, Wei W, Song LH. 2006; Protective effect of paeoniflorin on immunological liver injury induced by bacillus Calmette-Guerin plus lipopolysaccharide: modulation of tumour necrosis factor-alpha and interleukin-6 MRNA. Clin Exp Pharmacol Physiol. 33:332–339. DOI: 10.1111/j.1440-1681.2006.04371.x. PMID: 16620297.


28. Zhong SZ, Ge QH, Li Q, Qu R, Ma SP. 2009; Peoniflorin attentuates Abeta((1-42))-mediated neurotoxicity by regulating calcium homeostasis and ameliorating oxidative stress in hippocampus of rats. J Neurol Sci. 280:71–78. DOI: 10.1016/j.jns.2009.01.027. PMID: 19268972.

29. Zhou H, Bian D, Jiao X, Wei Z, Zhang H, Xia Y, He Y, Dai Y. 2011; Paeoniflorin protects against lipopolysaccharide-induced acute lung injury in mice by alleviating inflammatory cell infiltration and microvascular permeability. Inflamm Res. 60:981–990. DOI: 10.1007/s00011-011-0359-9. PMID: 21744312.


30. Wang Z, Liu Z, Yu G, Nie X, Jia W, Liu RE, Xu R. 2018; Paeoniflorin inhibits migration and invasion of human glioblastoma cells via suppression transforming growth factor β-induced epithelial-mesenchymal transition. Neurochem Res. 43:760–774. DOI: 10.1007/s11064-018-2478-y. PMID: 29423667. PMCID: PMC5842263.



31. Garber JC. Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research, Division on Earth and Life Studies, National Research Council of the National Academies. Committee for the update of the guide for the care and use of laboratory animals. Guide for the care and use of laboratory animals. 8th ed. 2011. National Academies Press;Washington, D.C.:
32. Rice JE 3rd, Vannucci RC, Brierley JB. 1981; The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 9:131–141. DOI: 10.1002/ana.410090206. PMID: 7235629.


33. Longa EZ, Weinstein PR, Carlson S, Cummins R. 1989; Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 20:84–91. DOI: 10.1161/01.STR.20.1.84. PMID: 2643202.


34. Garcia JH, Wagner S, Liu KF, Hu XJ. 1995; Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 26:627–634. discussion 635DOI: 10.1161/01.STR.26.4.627. PMID: 7709410.

35. Arteaga O, Álvarez A, Revuelta M, Santaolalla F, Urtasun A, Hilario E. 2017; Role of antioxidants in neonatal hypoxic-ischemic brain injury: new therapeutic approaches. Int J Mol Sci. 18:265. DOI: 10.3390/ijms18020265. PMID: 28134843. PMCID: PMC5343801.


36. Murugan M, Sivakumar V, Lu J, Ling EA, Kaur C. 2011; Expression of N-methyl D-aspartate receptor subunits in amoeboid microglia mediates production of nitric oxide via NF-κB signaling pathway and oligodendrocyte cell death in hypoxic postnatal rats. Glia. 59:521–539. DOI: 10.1002/glia.21121. PMID: 21319220.


37. Graham EM, Ruis KA, Hartman AL, Northington FJ, Fox HE. 2008; A systematic review of the role of intrapartum hypoxia-ischemia in the causation of neonatal encephalopathy. Am J Obstet Gynecol. 199:587–595. DOI: 10.1016/j.ajog.2008.06.094. PMID: 19084096.


38. Arteaga O, Revuelta M, Urigüen L, Álvarez A, Montalvo H, Hilario E. 2015; Pretreatment with resveratrol prevents neuronal injury and cognitive deficits induced by perinatal hypoxia-ischemia in rats. PLoS One. 10:e0142424. DOI: 10.1371/journal.pone.0142424. PMID: 26544861. PMCID: PMC4636303.

39. Zhao H, Yenari MA, Cheng D, Sapolsky RM, Steinberg GK. 2003; Bcl-2 overexpression protects against neuron loss within the ischemic margin following experimental stroke and inhibits cytochrome c translocation and caspase-3 activity. J Neurochem. 85:1026–1036. DOI: 10.1046/j.1471-4159.2003.01756.x. PMID: 12716434.


40. Qiu L, Zhu C, Wang X, Xu F, Eriksson PS, Nilsson M, Cooper-Kuhn CM, Kuhn HG, Blomgren K. 2007; Less neurogenesis and inflammation in the immature than in the juvenile brain after cerebral hypoxia-ischemia. J Cereb Blood Flow Metab. 27:785–794. DOI: 10.1038/sj.jcbfm.9600385. PMID: 16926844.


41. Wang X, Stridh L, Li W, Dean J, Elmgren A, Gan L, Eriksson K, Hagberg H, Mallard C. 2009; Lipopolysaccharide sensitizes neonatal hypoxic-ischemic brain injury in a MyD88-dependent manner. J Immunol. 183:7471–7477. DOI: 10.4049/jimmunol.0900762. PMID: 19917690.


42. Kaur C, You Y. 2000; Ultrastructure and function of the amoeboid microglial cells in the periventricular white matter in postnatal rat brain following a hypoxic exposure. Neurosci Lett. 290:17–20. DOI: 10.1016/S0304-3940(00)01306-9. PMID: 10925164.


43. Cunningham C. 2013; Microglia and neurodegeneration: the role of systemic inflammation. Glia. 61:71–90. DOI: 10.1002/glia.22350. PMID: 22674585.


44. Fernandez-Lizarbe S, Pascual M, Guerri C. 2009; Critical role of TLR4 response in the activation of microglia induced by ethanol. J Immunol. 183:4733–4744. DOI: 10.4049/jimmunol.0803590. PMID: 19752239.


45. Semenza GL. 2003; Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 3:721–732. DOI: 10.1038/nrc1187. PMID: 13130303.


46. Pugh CW, Ratcliffe PJ. 2003; Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 9:677–684. DOI: 10.1038/nm0603-677. PMID: 12778166.


47. Yao L, Kan EM, Lu J, Hao A, Dheen ST, Kaur C, Ling EA. 2013; Toll-like receptor 4 mediates microglial activation and production of inflammatory mediators in neonatal rat brain following hypoxia: role of TLR4 in hypoxic microglia. J Neuroinflammation. 10:23. DOI: 10.1186/1742-2094-10-23. PMID: 23388509. PMCID: PMC3575244.



48. Li Q, Verma IM. 2002; NF-kappaB regulation in the immune system. Nat Rev Immunol. 2:725–734. DOI: 10.1038/nri910. PMID: 12360211.

49. Shen W, Zhang C, Zhang G. 2002; Nuclear factor kappaB activation is mediated by NMDA and non-NMDA receptor and L-type voltage-gated Ca2+ channel following severe global ischemia in rat hippocampus. Brain Res. 933:23–30. DOI: 10.1016/S0006-8993(02)02291-6. PMID: 11929632.

50. Bianchi R, Giambanco I, Donato R. 2010; S100B/RAGE-dependent activation of microglia via NF-kappaB and AP-1 Co-regulation of COX-2 expression by S100B, IL-1beta and TNF-alpha. Neurobiol Aging. 31:665–677. DOI: 10.1016/j.neurobiolaging.2008.05.017. PMID: 18599158.

51. Back SA, Rosenberg PA. 2014; Pathophysiology of glia in perinatal white matter injury. Glia. 62:1790–1815. DOI: 10.1002/glia.22658. PMID: 24687630. PMCID: PMC4163108.



52. Segovia KN, McClure M, Moravec M, Luo NL, Wan Y, Gong X, Riddle A, Craig A, Struve J, Sherman LS, Back SA. 2008; Arrested oligodendrocyte lineage maturation in chronic perinatal white matter injury. Ann Neurol. 63:520–530. DOI: 10.1002/ana.21359. PMID: 18393269. PMCID: PMC3140464.



53. Inder TE, Volpe JJ. 2000; Mechanisms of perinatal brain injury. Semin Neonatol. 5:3–16. DOI: 10.1053/siny.1999.0112. PMID: 10802746.


54. Wang X, Hagberg H, Zhu C, Jacobsson B, Mallard C. 2007; Effects of intrauterine inflammation on the developing mouse brain. Brain Res. 1144:180–185. DOI: 10.1016/j.brainres.2007.01.083. PMID: 17320062.


Fig. 1
Neurobehavioral deficits following hypoxic ischemia. Values are represented as mean ± standard deviation, n = 6. p < 0.05 as determined by one-way ANOVA followed by DMRT analysis. H/I, hypoxia/ischemia; P, postpartum day. *Represents p < 0.05 vs. control; #represents p < 0.05 vs. H/I control. a-eRepresents mean values from different experimental groups that differ from each other at p < 0.05.
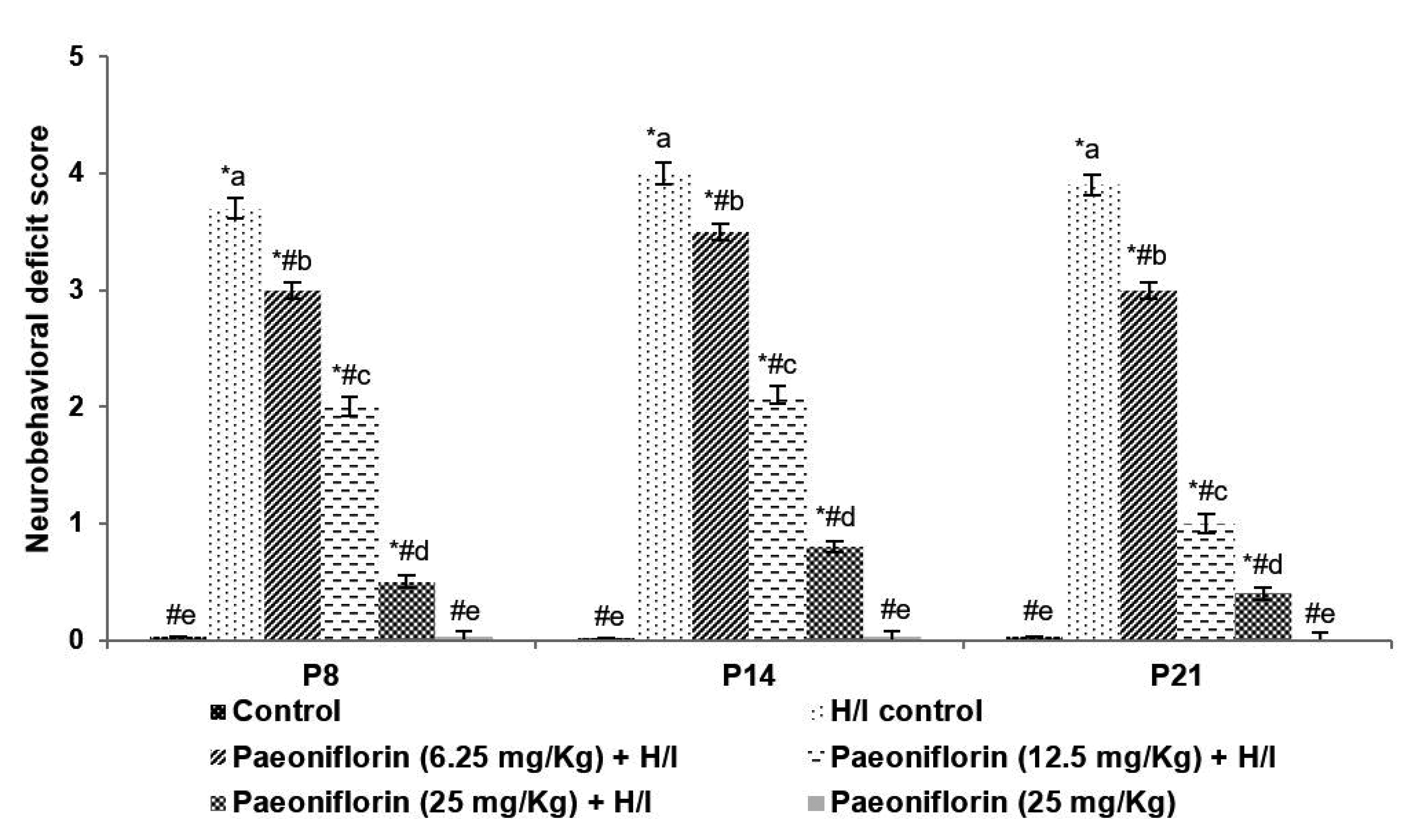
Fig. 2
Paeoniflorin reduced cerebral infarct area. Cresyl violet staining (×100) (A) and expressed infract volume (B). Values are represented as mean ± standard deviation, n = 6. p < 0.05 as determined by one-way ANOVA followed by DMRT analysis. H/I, hypoxia/ischemia; P, postpartum day. *Represents p < 0.05 vs. control; #represents p < 0.05 vs. H/I control. a-dRepresents mean values from different experimental groups that differ from each other at p < 0.05 (a-dControl, e-hH/I control, i-lpaeoniflorin [25 mg/kg]).

Fig. 3
Paeoniflorin reduced neuronal apoptosis. Tissue sections in TUNEL assay (A) and apoptotic cell count (B). Values are represented as mean ± standard deviation, n = 6. p < 0.05 as determined by one-way ANOVA followed by DMRT analysis. H/I, hypoxia/ischemia; P, postpartum day. *Represents p < 0.05 vs. control; #represents p < 0.05 vs. H/I control. a-dRepresents mean values from different experimental groups that differ from each other at p < 0.05 (a-cControl, d-fH/I control, g-ipaeoniflorin [25 mg/kg] + H/I, j-lpaeoniflorin [25 mg/kg]).

Fig. 4
Paeoniflorin regulated the expression of apoptotic proteins 24 h following hypoxic ischemia in P7 pups. (A) Western blotting and protein expression (B). Values are represented as mean ± standard deviation, n = 6. p < 0.05 as determined by one-way ANOVA followed by DMRT analysis. H/I, hypoxia/ischemia; P, postpartum day. *Represents p < 0.05 vs. control; #represents p < 0.05 vs. H/I control. a-eRepresents mean values from different experimental groups that differ from each other at p < 0.05 (L1, control; L2, H/I control; L3, paeoniflorin [6.25 mg/kg] + H/I; L4, paeoniflorin [12.5 mg/kg] + H/I; L5, paeoniflorin [25 mg/kg] + H/I; L6, paeoniflorin (25 mg/kg]).
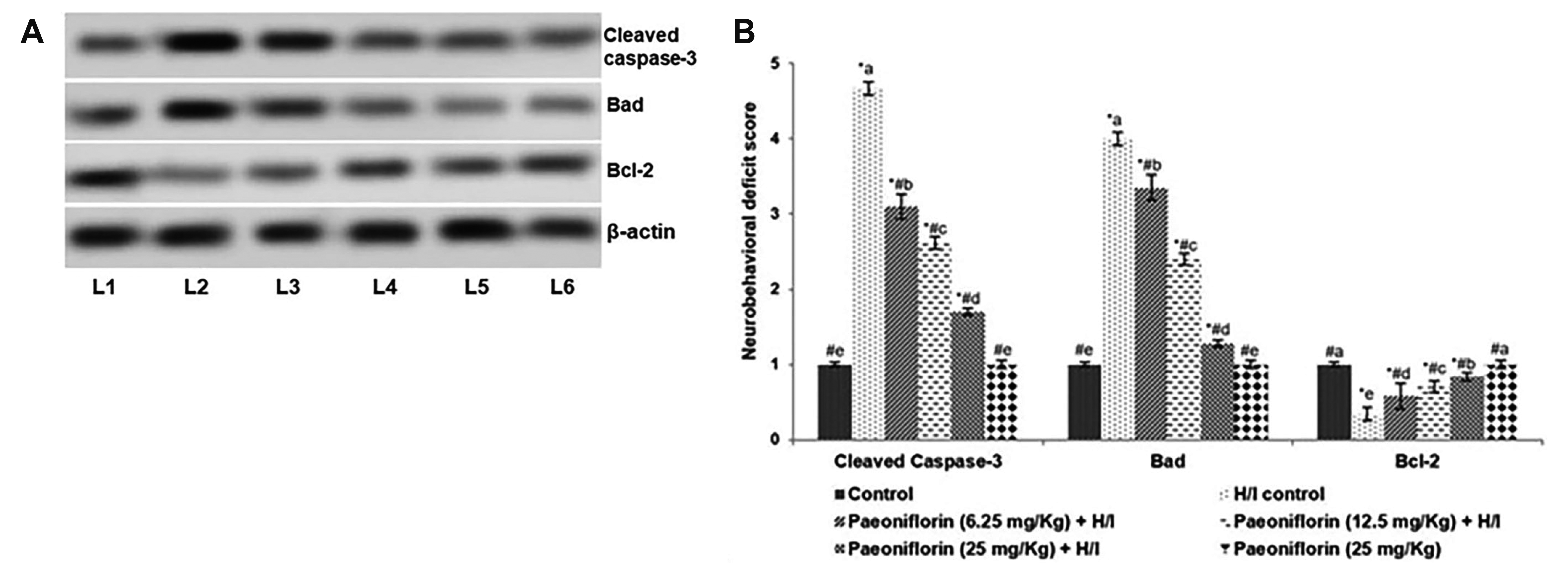
Fig. 5
Paeoniflorin reduces oxidative stress. Paeoniflorin reduced ROS generation following H/I (A) MDA levels (B) and improved GSH levels (C). Values are represented as mean ± standard deviation, n = 6. p < 0.05 as determined by one-way ANOVA followed by DMRT analysis. ROS, reactive oxygen species; MDA, malondialdehyde; GSH, glutathione; H/I, hypoxia/ischemia; P, postpartum day. *Represents p < 0.05 vs. control; #represents p < 0.05 vs. H/I control. a-eRepresents mean values from different experimental groups that differ from each other at p < 0.05.

Fig. 6
Paeoniflorin reduced microglial activation. Paeoniflorin reduced Iba-1 expression (A) microglial activation TLR4 (B) and also reduced microglial activation HIF-1α (C). Values are represented as mean ± standard deviation, n = 6. p < 0.05 as determined by one-way ANOVA followed by DMRT analysis. Iba-1, ionized calcium binding adaptor molecule 1; HIF-1α, hypoxia-inducible factor-1 alpha; H/I, hypoxia/ischemia; P, postpartum day. *Represents p < 0.05 vs. control; #represents p < 0.05 vs. H/I control. a-eRepresents mean values from different experimental groups that differ from each other at p < 0.05.

Fig. 7
Paeoniflorin regulated the expression of NF-κB following hypoxic ischemia. Values are represented as mean ± standard deviation, n = 6. p < 0.05 as determined by one-way ANOVA followed by DMRT analysis. TNF-α, tumor necrosis factor-α; H/I, hypoxia/ischemia; P, postpartum day. *Represents p < 0.05 vs. control; #represents p < 0.05 vs. H/I control. a-eRepresents mean values from different experimental groups that differ from each other at p < 0.05 (L1, control; L2, H/I control; L3, paeoniflorin [6.25 mg/kg] + H/I; L4, paeoniflorin (12.5 mg/kg) + H/I; L5, paeoniflorin (25 mg/kg) + H/I; L6, paeoniflorin (25 mg/kg]).

Fig. 8
Paeoniflorin reduced the levels of inflammatory cytokines. Values are represented as mean ± standard deviation, n = 6. p < 0.05 as determined by one-way ANOVA followed by DMRT analysis. IL-1β, interleukin-1β; TNF-α, tumor necrosis factor-α; H/I, hypoxia/ischemia; P, postpartum day. *Represents p < 0.05 vs. control; #represents p < 0.05 vs. H/I control. a-eRepresents mean values from different experimental groups that differ from each other at p < 0.05.
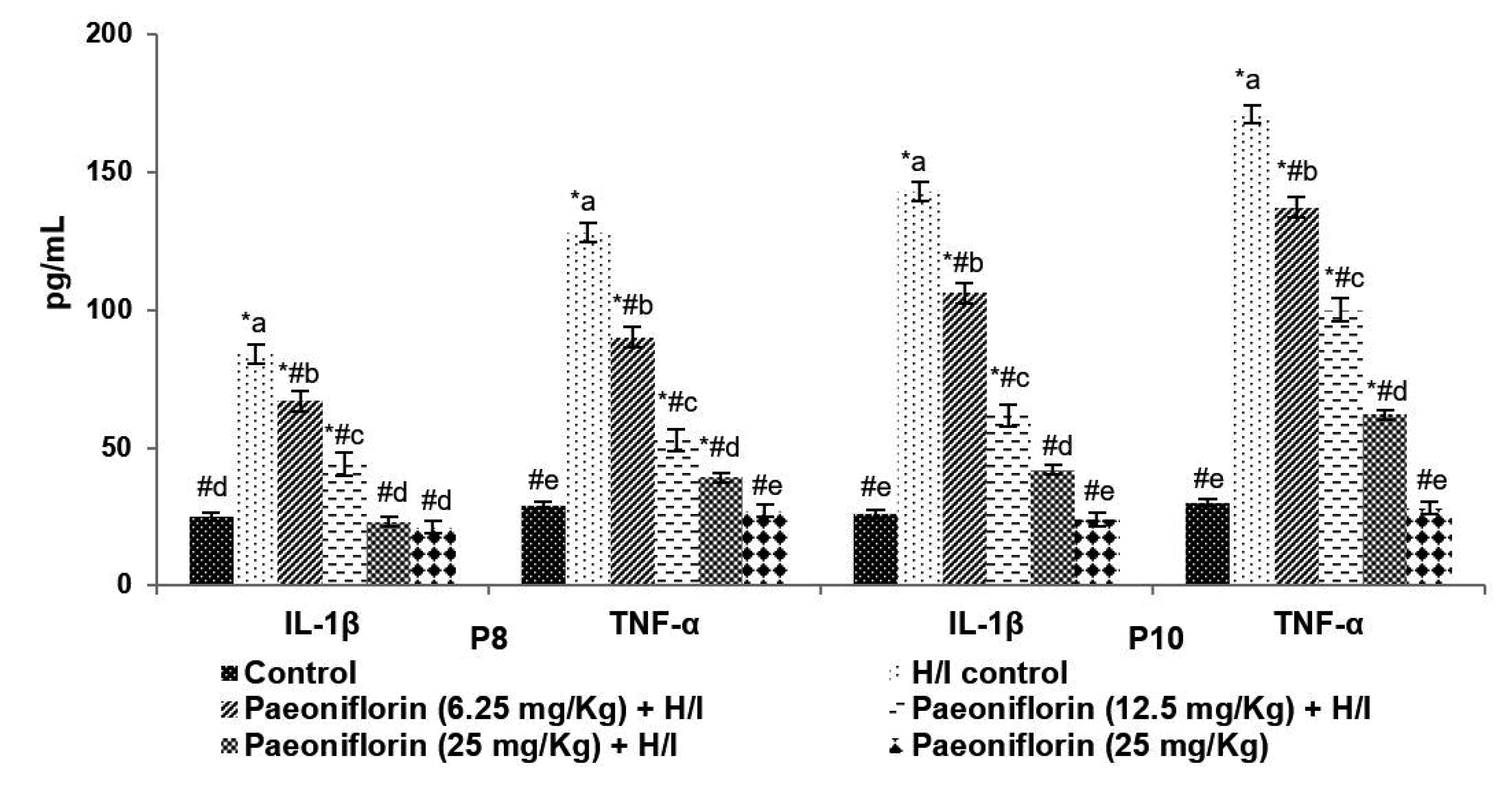
Fig. 9
Paeoniflorin improved MBP levels (A), O1 levels (B), and also O4 levels (C). Values are represented as mean ± standard deviation, n = 6. p < 0.05 as determined by one-way ANOVA followed by DMRT analysis. MBP, myelin binding protein; O, oligodendrocytes; H/I, hypoxia/ischemia; P, postpartum day. *Represents p < 0.05 vs. control; #represents p < 0.05 vs. H/I control. a-eRepresents mean values from different experimental groups that differ from each other at p < 0.05.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download